Attempts can be made to reduce non-food exposures (NFEs) in dietary intervention studies. However, it is impractical (and probably impossible) to eliminate them.
Plastic micro- and nanoparticles, as well as the chemical compounds used to produce, are ubiquitous in the environment and present formidable confounding factors that make it impossible to establish causal relationships between a single chemical and measured human health outcomes.
This ad-free article is made possible by the financial support of the
Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit.
Please consider making a tax-deductible donation for continued biomedical research.
That reality ranks among the top reasons that dormitory conditions offer the optimum solution for study results that can be both reproducible and clinically significant.
Reducing non-food exposures (NFEs)
Sathyanarayana et al. (2013) posited that substantial non-food exposures such as those with personal care products could mask BPA and phthalate reductions from dietary intervention.[xxxvii]
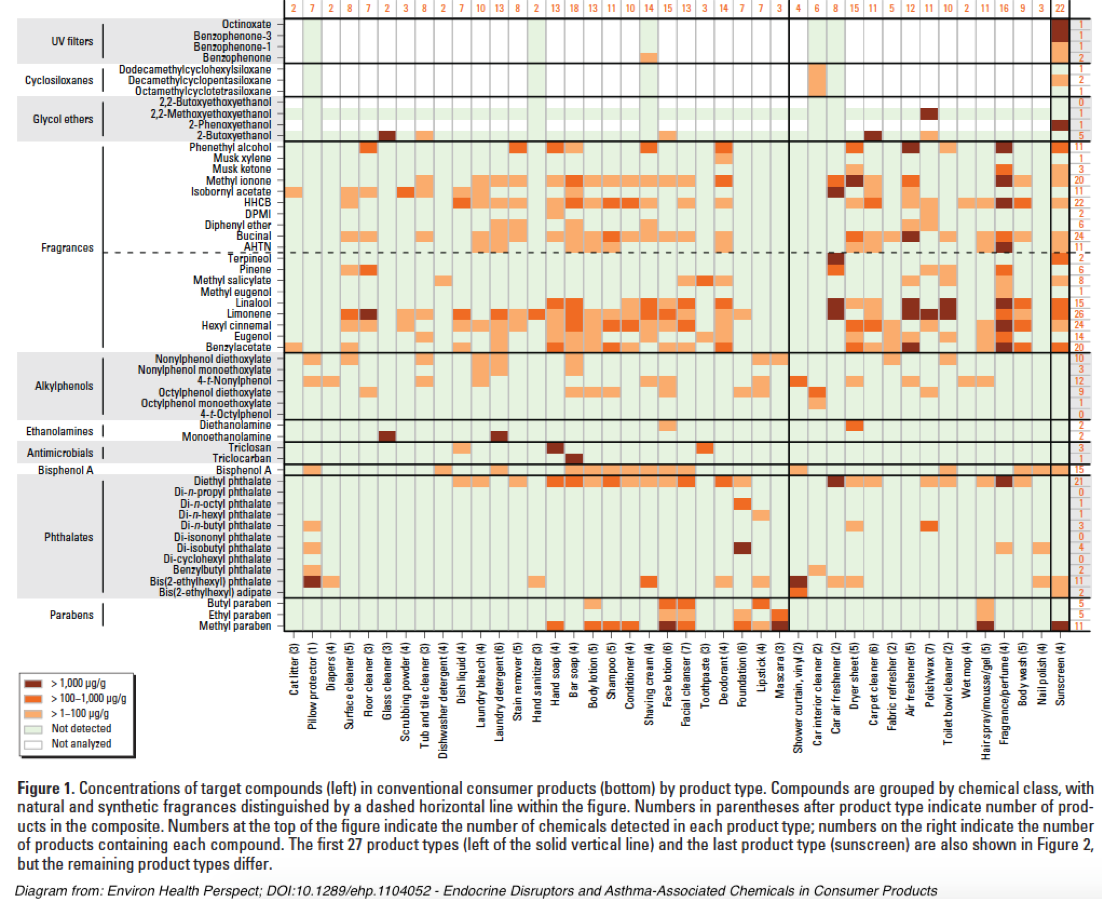
A quick picture of the complexity of NFEs can be illustrated by this diagram from Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products[xxxviii]:
An extensive discussion of NFE contamination sources may be accessed in Appendix 4.
Triaging NFE elimination
Given the impossibility of avoiding BPA, phthalates, and other harmful chemicals in the environment, reproducibility of studies must focus on the practical reduction of known exposures reducing those exposures that are:
- Most easily identified and,
- Practical to control,
- Likely to produce maximum reduction via minimum study subject effort, and
- Can be adequately documented and replicated.
An ideal scenario would be to conduct trials in a well-defined residential/dormitory setting where all study subjects experience near-identical environmental conditions for the entire term of the study.
However, expense and practicality dictate that many studies must engage subjects who will modify their diets while living in their own homes as well as engaged in multiple additional sites for work, school, shopping, and recreation.
Because of those practical limitations, investigators should — to the best of their ability — thoroughly document the environmental circumstances under which a dietary intervention is conducted. This should include questionnaires about known exposure sources for their:
- External environment (home, work, school, recreation including floor surfaces) and,
- Personal environment (pharmaceuticals, personal care products, appliances, fabrics for clothing and bedding, presence of plastic dental and other implants and prosthetics, etc.).
Participants with excessive and/or uncontrollable external environmental exposures should be excluded.
Study subjects should then be counseled on contamination sources and given a checklist of specific controllable sources to avoid during the trial.
Investigators should schedule frequent reminders and follow-ups with each participant to assess and assure the best possible compliance.
As a potential starting point for assessing personal environmental exposures, investigators should consider the EDC Footprint Calculator developed by the Pennsylvania State University Extension Service. While this Excel version was designed to offer an indication of contamination products found in municipal wastewater and is not all-inclusive, it could be modified and updated to better serve the purposes of this study or future efforts.
Personal environment controls
As the above chart from “Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products” illustrates, most personal care products contain relevant chemical contaminants that can potentially confound this study’s chosen health effects markers.
Select three-day reduction actions for the personal environment:
NOTE: To make these reductions possible and assure the greatest possibilities for reproducibility, study subjects must be healthy, non-smoking adults without recent dental work or invasive medical procedures. Subjects should not have body fat of more than 32% for women and 25% for men. Subjects should not be taking any medicines or dietary supplements other than low-dose or 325 mg aspirin.
Additional requirements should include:
- Use recommended toothpaste without Triclosan and reduced or zero levels of contaminants.
- No mouthwash.
- Uncoated nylon fiber dental floss (no plastic strips).
- No hand creams or lotions.
- No perfumes or any other products containing fragrances.
- Use recommended shampoo with reduced or zero-levels of contaminants.
- Use recommended hand soap with reduced or zero-levels of contaminants.
- No cosmetics.
- No hair color treatment.
- No teeth whitener.
- No hand sanitizers.
- Use recommended deodorant.
- No contact lenses. Use of eyeglasses only.
- No contact with cash register receipts.
- No hair spray, gel, or other treatment.
- No body wash.
- No sunscreen.
- No nail polish.
- No Tshirts, sweatshirts, or other clothing with printed designs.
In addition, subjects must note the synthetic composition of clothing and bedding items. That data may be valuable for investigating possible confounding factors.
External environment controls
In addition to food contamination issues, home, workplace, and public spaces also provide significant — but widely variable — exposure to humans to endocrine disruptors, CECs and, other environmental chemicals[xxxix].
Thus, additional requirements should include:
- Do not use inkjet printers, gel rollerball pens, or ballpoint pens.
- No use of volatile adhesives such as rubber cement or spray cans.
- Use no household cleaners.
- No reusable, non-paper grocery/shopping bags with printed designs.
- No plastic wallets/purses/backpacks.
- Inform investigators if beds or pillows have plastic covers.
- No air fresheners.
- Avoid areas irrigated with recycled wastewater.
- Do not get into a vehicle until the interior has been thoroughly ventilated with outside air.
- Ride in vehicles with all windows open. Do not use an air freshener. Avoid new vehicles and those whose interiors have been recently cleaned commercially.
- Wear only those clothes that have been washed with phthalate-free laundry detergents.
- Unless a phthalate-free laundry detergent has been used previously, run two loads of towels, then dry.
- If fabric softeners have been used in the dryer, run two additional loads of towels after decontaminating the washer and dry them in the dryer to reduce accumulated phthalate levels.
- Avoid bathing in homes whose water supply is delivered with plastic plumbing: PVC, Pex, etc.
Dust particles
Household dust presents a significant confounding factor because it is the residual detritus from many environmental sources shown to contain BPA, phthalates, and other chemicals relevant to this study: Phthalates in Indoor Dust and Their Association with Building Characteristics[xl]. That study contains a home materials inventory that will be adapted for the study subject questionnaire. See also PM 2.5[xli].
Reduction actions for dust particles:
Frequent and thorough cleaning (before the start of phase 4) of all surfaces (floor, walls, ceilings) with a mop dampened with water only. No cleaning compounds which may introduce their own unique, unknown, and uncontrollable contaminants.
If the cleaning/dusting is performed by the test subject, then tasks should be performed wearing an N-95/p100 mask/filter. Significantly, masks of that particular quality do not filter all of the most hazardous particulates — those in the 2.5 microns (PM 2.5) and smaller size.
The PM 2.5 size class is most likely to reach and be retained by alveoli and thus have direct access to the bloodstream.
As a consequence, PM 2.5 is implicated in a wide range of serious health effects[xlii],[xliii],[xliv].
The type and age of the HVAC system should be noted as well as the type of filter used and the ducting materials (metal versus plastic).
Immediately after dusting, the HVAC filter should be changed and the system fan run continuously for four hours to collect as much dust as possible which has been stirred up from cleaning. A fresh filter should then be installed.
The slightly-used filter may be preserved for future use if handled gently and immediately inserted into a suitably-sized plastic bag. Investigators note the ironic necessity to use plastic to help protect against plastic.
Study subjects who already have a HEPA-class air purifier should report the make, model, capacity, and whether it is portable or installed with the HVAC system. If portable, the location should be noted along with the distance from the HVAC air return.
More supplemental references may be found in Appendix 5.
[i] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138.
[ii] Serrano, S.E., Braun, J., Trasande, L., Dills, R., and Sathyanarayana, S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health 13: 43.
[iii] Guart, A., Bono-Blay, F., Borrell, A., and Lacorte, S. 2011. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Additives & Contaminants Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 25(5): 676-685.
[iv] Bhunia, K., Sablani, S.S., Tang, J., and Rasco, B. 2013. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Comprehensive Reviews in Food Science and Food Safety 12(5): 523-545.
[v] Bang, D.Y., Kyung, M., Kim, M.J., Jung, B.Y., Cho, M.C., Choi, S.M., Kim, Y.W., Lim, S.K., Lim, D.S., Won, A.J., Kwack, S.J., Lee, Y., Kim, H.S., and Lee, B.M. 2012. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Comprehensive Reviews in Food Science and Food Safety 11(5): 453-470.
[vi] Groh, K.J., Geuke, B., and Muncke, J. 2017. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food and Chemical Toxicology 109(1): 1-18.
[vii] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103.
[viii] Schecter, A., Lorber, M., Guo, Y., Wu, Q., Yun, S.H., Kannan, K., Hommel, M., Imran, N., Hynan, L.S., Cheng, D., Colacino, J.A., and Birnbaum, L.S. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environmental Health Perspectives 121(4): 473-479.
[ix] Cariou, R., Larvor, F., Monteau, F., Marchand, P., Bichon, E., Dervilly-Pinel, G., Antignac, J-P., and Le Bizec, B. 2016. Measurement of phthalates diesters in food using gas chromatography-tandem mass spectrometry. Food Chemistry 196: 211-219.
[x] Van Holderbeke, M., Geerts, L., Vanermen, G., Servaes, K., Sioen, I., De Henauw, S., and Fierens, T. 2014. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environmental Research 134: 345-352.
[xi] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138.
[xii] Cirillo, T., Latini, G., Castaldi, M.A., Dipaola, L., Fasano, E., Esposito, F., Scognamiglio, G., Di Francesco, F., and Cobellis, L. 2015. Exposure to di-2-ethyhexyl phthalate, di-N-butyl phthalate and bisphenol A through infant formulas. Journal of Agricultural and Food Chemistry 63(12): 3303-3310.
[xiii] Fierens, T., Vanermen, G., Van Holderbeke, M., De Henauw, S., and Sioen, I. 2012. Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology 50(12): 4428-4435.
[xiv] Ionas, A.C., Dirtu, A.C., Anthonissen, T., Neels, H., and Covaci, A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environment International 65: 54-62.
[xv] Hamrick, K., and McClelland, K. 2016. Americans’ Eating Patterns and Time Spent on Food: The 2014 Eating & Health Module Data. Economic Information Bulletin No. (EIB-158); 51pp.
[xvi] Guart, A., Wagner, M., Mezquida, A., Lacorte, S., Oehlmann, J., and Borrell, A. 2013. Migration of plasticizers from TritanTM and polycarbonate bottles and toxicological evaluation. Food Chemistry 141(1): 373-380.
[xvii] Bittner, G.D., Yang, C.Z., and Stoner, M.A. 2014. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environmental Health 13: 41.
[xviii] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103.
[xix] Dolinoy, D.C., Huang, D., and Jirtle, R.L. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. PNAS 104(32): 13056-13061.
[xx] Tarapore, P., Ying, J., Ouyang, B., Burke, B., Bracken, B., and Ho, S.M. 2014. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3): e90332.
[xxi] Pepys, M, B., and Hirschfield, G.M. 2003. C-reactive protein: A critical update. The Journal of Clinical Investigation 111(12): 1805-1812.
[xxii] Gao, H., Yang, B-J., Li, N., Feng, L-M., Shi, X-Y., Zhao, W-H., and Liu, S-J. 2015. Bisphenol A and Hormone-Associated Cancers: Current Progress and Perspectives. Medicine 94(1): e211.
[xxiii] Tarapore, P., Ying, J., Ouyang, B., Burke, B., Bracken, B., and Ho, S.M. 2014. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3): e90332.
[xxiv] Labbé, D.P., Zadra, G., Ebot, E.M., Mucci, L.A., Kantoff, P.W., Loda, M., and Brown, M. 2014. Role of diet in prostate cancer: the epigenetic link. Oncogene 34: 4683-4691.
[xxv] Fisher, M., Arbuckle, T.E., Mallick, R., LeBlanc, A., Hauser, R., Feeley, M., Koniecki, D., Ramsay, T., Provencher, G., Bérubé, R., and Walker, M. 2015. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology 25: 231-239.
[xxvi] Stahlhut, R.W., Welshons, W.V., and Swan, S.H. 2009. Bisphenol A data in NHANES suggests longer than expected half-life, substantial nonfood exposure, or both. Environmental Health Perspectives 117(5): 784-789.
[xxvii] Song, Y., Hauser, R., Hu, F.B., Franke, A.A., Liu, S., and Sun, Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International Journal of Obesity 38(12): 1532-1537.
[xxviii] Fisher, M., Arbuckle, T.E., Mallick, R., LeBlanc, A., Hauser, R., Feeley, M., Koniecki, D., Ramsay, T., Provencher, G., Bérubé, R., and Walker, M. 2015. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology 25: 231-239.
[xxix] Larsson, K., Björklund, K.L., Palm, B., Wennberg, M., Kaj, L., Lindt, C.H., Jönsson, B.A.G., and Berglund, M. 2014. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environment International 73: 323-333.
[xxx] Hutter, K.P., Kundi, M., Hohenblum, P., Scharf, S., Shelton, J.F., Piegler, K., and Wallner, P. 2016. Life without plastic: A family experiment and biomonitoring study. Environmental Research 150: 639-644.
[xxxi] Ji, K., Lim Kho, Y., Park, Y., and Choi, K. 2010. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: a pilot study with “Temple Stay” participants. Environmental Research 110(4): 375-382.
[xxxii] Galloway, T.S., Baglin, N., Lee, B.P., Kocur, A.L., Shepherd, M.H., Steele, A.M., BPA Schools Study Consortium, and Harries, L.W. 2018. An engaged research study to assess the effect of a “real-world” dietary intervention on urinary bisphenol A (BPA) levels in teenagers. BMJ Open 8: e018742.
[xxxiii] Rudel, R.A., Gray, J.M., Engel, C.L., Rawsthorne, T.W., Dodson, R.E., Ackerman, J.M., Rizzo, J., Nudelman, J.L., and Brody, J.G. 2011. Food packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate exposure: Findings from a dietary intervention. Environmental Health Perspectives 119(7): 914-920.
[xxxiv] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxv] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxvi] Xue, J., Liu, W., and Kannan, K. 2017. Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environmental Science & Technology 51(9): 5279-5286.
[xxxvii] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxviii] Dodson, R.E., Nishioka, M., Standley, L.J., Perovich, L.J., Brody, J.G., and Rudel, R.A. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental Health Perspectives 120(7): 935-943.
[xxxix] Song, Y., Hauser, R., Hu, F.B., Franke, A.A., Liu, S., and Sun, Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International Journal of Obesity 38(12): 1532-1537.
[xl] Bornehag, C-G., Lundgren, B., Weschler, C.J., Sigsgaard, T., Hagerhed-Engman, L, and Sundell, J. 2005. Pthalates in indoor dust and their association with building characteristics. Environmental Health Perspectives 113(10): 1399-1404.
[xli] Marshall, J. 2013. PM 2.5. PNAS 110(22): 8756.
[xlii] Schwartz, J., Laden, F., and Zanobetti, A. 2002. The concentration-response relation between PM(2.5) and daily deaths. Environmental Health Perspectives 110(10): 1025-1029.
[xliii] Pope III, C.A., Ezzati, M., Cannon, J.B., Allen, R.T., Jerrett, M., and Burnett, R.T. 2018. Mortality risk and PM2.5 air pollution in the USA: an analysis of a national prospective cohort. Air Quality, Atmosphere & Health 11(3): 245-252.
[xliv] Franklin, M., Zeka, A., and Schwartz, J. 2007. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of Exposure Science & Environmental Epidemiology 17: 279-287.