This post written by Study Designer Lewis Perdue. Following is the text summary of a study developed by Co-Principal Investigator Lewis Perdue to be co-conducted with Victor Reus, M.D., The Distingushed Professor of Medicine at UCSF.
The study is currently being evaluated by the University of California San Francisco School of Medicine, Institutional Review Board. Funding for the study is being lead by the Center For Research on Environmental Chemicals in Humans — a 501(c)(3) non-profit affiliated with the University of California San Francisco School of Medicine.
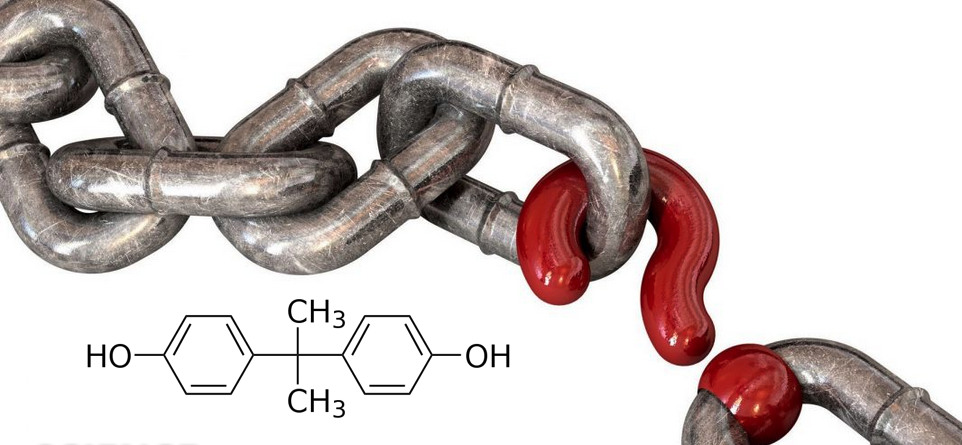
The First Double-Blind, Randomized, Controlled Human Clinical Trial Designed To Bring Clarity To The Bisphenol Controversy
The U.S. Food and Drug Administration has stated that common environmental chemicals such as BPA are safe for human consumption at levels commonly found in food, drink and the environment. That notion has been challenged by researchers at a number of academic laboratories. The biggest source of this confusion is the fact that the FDA’s advice is based on studies conducted using mice as test subjects. Mice, however, are not humans and often do not react to chemicals in the same way. This means that those studies are non-causal with respect to humans. In addition, the handful of studies done regarding humans are methodologically flawed, marred by confounding factors, and therefore are non-reproducible and non-causal. Valid science demands causal outcomes that are reproducible. In this respect, U.S. federal regulation of many environmental chemicals is invalid. The researchers in this study have designed a causal, reproducible protocol to determine whether humans react the same way to BPA (Bisphenol A) and two extensively used analogues, BPF and BPS.
Click here to read the background about how government science has created uncertainty about the controversy and hindered the public and medical practicioners from knowing the risks.
This ad-free article is made possible by the financial support of the Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit. Please consider making a tax-deductible donation for continued biomedical research.
Hypothesis:
Investigators hypothesize that administering reagents Bisphenol A, Bisphenol F, and Bisphenol S to subjects at a level consistent with current United States individual exposures, not to exceed the Oral Reference Dose (RfD) of 50 μg/kg/day for BPA as established by the U.S. Department of Health and Human Services and the Environmental Protection Agency will have a measurable effect on serum inflammation and metabolism biomarkers
Summary
This is an eight-day, seven-night, blinded clinical study of 40 healthy, physically fit, male, human adults aged 21 to 35 who will spend eight days in an environmentally controlled dormitory setting. Test subjects will be fed specially prepared meals and beverages designed to minimize environmental chemicals and substances found in pre-prepared and ultra-processed foods. Test subjects will consume the same specially prepared foods and beverages which will be be dosed with amounts of common bisphenols BPA, BPF and BPS to simulate the average American’s daily exposure. Controls will receive identical meals and beverages free of those compounds. Daily doses will not exceed the Oral Reference Dose (RfD) of 50 μg/kg/day for BPA as established by the U.S. Department of Health and Human Services (https://www.fda.gov/media/90124 /download accessed 071623) and the Environmental Protection Agency (https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=356 accessed 071623) One week prior to the start of the trial, all subjects will be equipped with Apple Watches for baseline and continuous, recorded monitoring of sleep cycles, heart rate, and other vital signs which may be valuable in determining the effects of the dormitory setting. Subjects will also be provided with a networked tablet for use recording exercise activities and personal notes relevant to the study. Final acceptance of test subjects for inclusion will require, after ICF, a blood draw as described below. All subjects will be given a copy of the California Experimental Subject’s Bill of Rights before they sign the consent form. After check-in on the first day of the trial (after fasting other than water after midnight) subjects will undergo a blood draw and urine sample, as described below. The initial study blood sample will also test for a comparison with the initial blood draw taken for qualification to be accepted for the study. An Informed Consent Agreement will inform subjects of the substances being examined in the blood draws which will not exceed two tablespoons (30 milliliters). at each draw. Substances to be examined include: Health Indicators:
- hsCRP
- TNF-alpha
- interleukin-6
- A1C
- ghrelin (metabolic)
- FGF-21.
- cortisol,
- testosterone,
- PSA (Prostate Specific Antigen)
All analyte concentrations must be within 30% of the midrange of UCSF laboratory reference ranges.
Toxicology Screen
Substances to be examined will be those specified in The UCSF toxicology screen (https://www.ucsfhealth.org/medical-tests/toxicology-screen) and may include:
- Alcohol (ethanol) — “drinking” alcohol
- Amphetamines
- Antiepressants
- Barbiturates and hypnotics
- Benzodiazepines
- Cocaine
- Flunitrazepam (Rohypnol)
- Gamma hydroxybutyrate (GHB)
- Marijuana
- Narcotics
- Non-narcotic pain medicines, including acetaminophen and anti-inflammatory drugs Phencyclidine (PCP)
- Phenothiazines (antipsychotic or tranquilizing medicines)
- Prescription medicines, any type
All blood samples will be tested for BPA, BPF and BPS and their glucuronidation products. Cortisol will be included as a potential biomarker related to the possible individual psychological stress as the result of social interactions with strangers in a confined dormitory environment. Because bisphenols are estrogen mimics, testing will include testosterone and PSA (Prostate Specific Antigen). Urine samples will be analyzed for BPA, BPF and BPS and their glucuronidation products. Blood sampling will be repeated on days 2, 5 and 8 of the study; urine will be collected on a daily basis. Subjects will be removed from the study if the presence of any tox screen analyte is found in blood samples.
Schedule
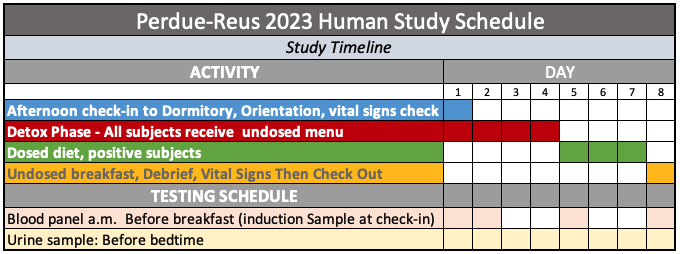
After check-in on Day 1, participants will replace their clothing with garments and shoes made of all natural fibers and materials. This is in keeping with the overall minimization of microplastics in the study environment including HEPA air filtration, fabrics, furnishings, sheets, towels and other potentially affected items. On days 1-4, All participants will receive bisphenol-free, un-dosed foods. On days 5-8:
- 10 of the participants will receive foods dosed with BPA.
- 10 of the participants will receive foods dosed with BPF
- 10 of the participants will receive foods dosed with BPS
- 10 of the participants (controls) will receive undosed foods.
Dosing will be double blinded. To simplify meal preparation, and dosing, subjects will eat the same breakfast, and the same lunch, the same dinner each day. Subjects will be required to consume all of their mealtime food and beverages. Un-dosed snacks will be provided as desired by each subject and the amounts recorded. Carbon-filtered water will be provided and consumed as needed. On days 2, 5 and 8, all participants will undergo a blood draw before breakfast. Urine samples will be taken before bedtime on all days. Subjects will be required to adhere to the same sleep and wake times as set by the trial venue. Substantial variance may result in disqualification from trial. Meals will be sourced, prepared and served in accordance with tested protocols described at this source: Detailed parameters of intervention diet selections. https://stealthsyndromesstudy.com/?p=12. Accessed June 3, 2023. The physical environment of the subjects will be controlled to comply with the tested standards at this source: https://www.stealthsyndromesstudy.com/?p=1507. Accessed June 3, 2023.
More Background
Public awareness and regulatory oversight of the harms from ubiquitous environmental chemicals such as bisphenols and phthalates have gone mostly unrecognized by the general public because of non-reproducible and non-causal investigations in humans, and additional fatal flaws in animal model investigations. Two recent studies [1],[2],[3] that attempted to address this issue have advanced this knowledge, but still fell short due to unanticipated issues addressed by this proposed investigation. This proposed study employs a tightly controlled dormitory setting with strict environmental conditions for carefully vetted human subjects, along with the dosing of three extensively used bisphenols at or below regulatory maximum daily tolerable doses designed to advance the lessons of previous studies. The confusing replication and causality issues are particularly serious because environmental chemicals are widespread in the U.S[4]. More than 86,000 chemicals have been approved for use in the United States[5], and at least 4,000 of those are found in plastic-based food contact materials.[6],[7],[8] One of the most pervasive of these chemicals, Bisphenol A (BPA) is present in approximately 97% of the U.S. population.[9],[10] This has caused widespread misinformation concerning high-profile issues such as plastic-derived food (PDC) contaminants, the nutritional benefits of natural foods, and assessing harms from ultra-processed foods. The situation is most prominently illustrated in the case of the extensively studied Bisphenol A (BPA). Non-causal studies have associated BPA and other PDCs with cancer[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], cardiovascular disorders10,[21],[22],[23],[24],[25],[26],[27],[28] ,obesity[29],[30],[31],[32],[33],[34],[35], type 2 diabetes31,[36],[37],[38], metabolic syndrome27,28,29,[39],[40], neurological and behavioral disorders[41],[42] also including Alzheimer’s Disease10,36,[43],[44],[45],[46],[47], as well as reproductive11,[48],[49],[50],[51],[52],[53],[54],[55], developmental disorders11,[56],[57],[58],[59],[60],[61] and allergies[62],[63],[64],[65],[66],[67],[68],[69]. PDCs such as BPA are classified as endocrine disrupters, even in low-level concentrations.7,[70] More significantly, in a 10-year observational study of 3,883 adults in the United States, participants with higher urinary Bisphenol A levels were at a 49% greater risk for death from all causes.[71] What is more, one recent investigation[72] offers evidence that two Bisphenol analogues — BPF and BPS — are increasingly being used instead of BPA despite indications that both are as hazardous (or more so) than BPA[73],[74],[75]. This is known as a regrettable substitution[76],[77],[78]. Following that review, investigators believe these results may confirm speculation in many published studies that product manufacturers had engaged in regrettable substitutions[79],[80] by reducing BPA usage, and substituting Bisphenol analogues which appear to equal or exceed BPA human toxicity73,[81],[82],[83],[84],[85],[86],[87],[88],[89],[90]. The confusion based on issues of replication and causality in nutrition and regulatory science gives free reign to misinformation, political and public manipulation by vested interests, and frustration by clinicians who are on the battle lines of consumers’ questions. The inconsistency has led one major university study[91] to declare: “With this type of data, it is difficult to draw a meaningful, intellectually honest conclusion.” What is more, this situation may have wasted at least $28 billion annually in scientific research[92], and created a lack of trust for science among the more than 50% U.S. population[93] who object to animal experimentation. The inability of most scientific studies to be replicable or reproducible has also led some scientists to assert that there is a reproducibility crisis. A 2016 survey of 1,500 scientists reported that “70% of researchers have tried and failed to reproduce another scientist’s experiments.”[94] This issue is a long standing, chronic problem.[95] In the case of environmental chemical regulation, the problem is further aggravated by the divisiveness caused by the U.S. Food and Drug Administration’s conduct of a study marred by numerous methodological flaws[96],[97],[98],[99], the “core” CLARITY-BPA[100] study, extrapolating from its murine model, found no human harms from current exposures of Bisphenol A. Significantly, the FDA’s core CLARITY-BPA relied on a set of protocols known as Good Laboratory Practices (GLP) which were developed not to create protocols for replicable, studies but as a record-keeping device to prevent fraud on the part of corporate and other private labs.[101],[102],[103] Despite the fact that the FDA version is not replicable, causal, peer-reviewed, or published in scientific journals, FDA pointedly ignored its agreement to consider, in its regulatory actions, the work of 14 studies by academic laboratories working with CLARITY-BPA, that found harms in current exposures. Also not considered by the FDA in its CLARITY-BPA study are more than 8,000 published, peer-reviewed academic studies[104] detailing the effects of BPA. This absence was publicly called out by many of the academic researchers.96 According to a statement[105] on behalf of the Endocrine Society, an international professional organization with some 18,000 members including researchers and clinicians, the FDA’s version of CLARITY-BPA was premature and incorrectly concentrated on gross anatomy and traditional toxicology and failed to account for BPA’s significant endocrine-disrupting harms. The statement was backed up with a published article reviewing the results of CLARITY’s academic papers.[106] This confusion has significant consequences among members of the public who are ill-equipped to factually reconcile the dramatically differing opinions between government and academic researchers. What complicates matters even further the fact that animal study results translate poorly to humans. More than 90 percent of murine-based trials of promising pharmaceuticals fail to receive government approval because of poor performance in human trials.[107] Because of this, failure direct, clinical human experimental data is vital to providing replicable, causal outcomes. To develop direct human-relevant data, human dietary intervention studies have attempted to shed light on the controversy. Unfortunately, dietary intervention efforts thus far have not demonstrated causality or replicability because of methodological issues with food complexity, subject environmental control, memory, survey, and other confounding factors. Defining Replicability &
Reproducibility
Complicating the assessment of the issue is a common failure fail to differentiate between reproducible and replicable.[108] While reproducibility focuses on the ability to reproduce the same results using the same data and methods, replicability strives to obtain similar results using different data or methods. Of the two, reproducibility strives for a higher level of trust. Adding to the discussion, some investigators have struggled with nomenclature and offered the suggestion that there should be even finer degrees of differentiation.[109] In that publication, the authors argue for replicability separately defined by methods and by results: “Methods reproducibility refers to the provision of enough detail about study procedures and data so the same procedures could, in theory or in actuality, be exactly repeated.” In addition, “Results reproducibility” (previously described as replicability) refers to obtaining the same results from the conduct of an independent study whose procedures are as closely matched to the original experiment as possible.” Note that these definitions, while novel, deviate from the most restrictive definitions.
“Big Idea” Reproducibility
An even looser definition for reproducibility takes an even higher-level view: “The inherent variability in biological systems means there is no expectation that results will necessarily be precisely replicated. So it is not reasonable to expect that each component of a research report will be replicated in perfect detail. However, it seems completely reasonable that the one or two big ideas or major conclusions that emerge from a scientific report should be validated and with-stand close interrogation.”[110] Regardless of terminology, the lack of reproducibility or replicability has created a panoply of conflicting results plagued by equivocal and often contradictory results, replication failures, and significant barriers to translating results into valid clinical recommendations for the best personal health. That situation is further clouded because, thus far, a search of the scientific literature indicates that human nutrition studies as a whole are neither replicable nor causal because they are based on observational or prospective studies.
The Clinician’s Dilemma
This spills over into the relationships between health professionals and their clients when they are faced with a steady diet of popular articles, such as 5 Foods We Thought Were Bad For Us, Now Turn Out to Be Good[111]. The same ambivalence spills over into the scientific literature where one study finds evidence for the healthy consumption of nuts[112], and another is not so sure: “Should we go nuts about nuts?”[113] The author comments of one published study exemplifies clinician frustration: “A significant positive effect of the interventions on weight was reported by all study types. The meta-analysis showed that lifestyle interventions achieved weight and waist circumference reductions after one year. However, no clear effects on biochemical or clinical parameters were observed,… Lifestyle interventions for patients at high risk of diabetes, delivered by a variety of healthcare providers in routine clinical settings, are feasible but appear to be of limited clinical benefit one year after intervention. Despite convincing evidence from structured intensive trials, this systematic review showed that translation into routine practice has less effect on diabetes risk reduction.”[114]
What factors influence trust among the citizenry?
“An ASN-commissioned, independent Advisory Committee comprehensively reviewed the literature and available public surveys about the public’s trust in nutrition science and the factors that influence it and conducted stakeholder outreach regarding publicly available information. The Committee selected 7 overlapping domains projected to significantly influence public trust: 1) conflict of interest and objectivity; 2) public benefit; 3) standards of scientific rigor and reproducibility; 4) transparency; 5) equity; 6) information dissemination (education, communication, and marketing); and 7) accountability.”[115]
Restrictive Reproducibility For This Study
Investigators in this study have opted to conduct the present study to adhere to the most restrictive definition which we believe will enable the best possible results for replication: “In the most restrictive and precise use of the term, experimental results are replicated when an independent group performs the same experiment, under the same conditions and using the same reagents, then finding the same results.”[116]
Scientific Logic For Selection Of Human Subjects
Subjects for this study will be humans. In order to create replicable, causal science that is valid for both human environmental chemical regulation and evidence-based clinician recommendations, several fundamental characteristics must be met. Experimental studies done with a murine model are not causal with reference to humans. This is evidenced by the fact that most promising pharmaceuticals that show great promise and success with rats or mice ultimately fail in human trials. Pharmaceutical studies are among the most demanding scientific investigations because the results can be a matter of life or death. Despite thus, 92% of drugs deemed safe and effective in animals, fail when tested in humans[117]. While the murine model[118] can be invaluable in the preliminary stages[119] of investigating pharmaceuticals and toxic chemicals, there exists a wide variety of confounding factors inherent in the use of rodents as subjects.[120] Trust Logic Study outcome issues are not the only imperatives to using humans as test subjects. Ample research indicates that the general public trusts human research more and tends to find the use of animals unacceptable – further complicating trust in a study’s outcomes.[121],[122],[123],[124],[125]
Addressing Nuremberg’s legacy
The lack of controlled-dose human studies with environmental chemicals has been attributed to a Nuremberg-based ethical reluctance to expose human to harmful substances.[126],[127],[128],[129] Human experimentation ethics: Industry and federal government compliance? The Nuremberg ethical argument, however, fails because humans are already legally exposed to bisphenols and other plastic-derived chemicals with scores of other potentially harmful chemicals as evidenced by the National Health and Nutrition Examination Survey (NHANES)[130],[131],[132],[133] and numerous studies confirming chronic Bisphenol A levels in human serum and urine.[134],[135],[136]
Human Testing For Pharmacokinetics
A literature search did reveal a handful of investigations in which human test subjects were directly administered Bisphenol A in order to assess BPA pharmacokinetics (but not health effects).[137],[138]
Alternatives to Dosing Are Impractical
The application of this classic experimental design to a dietary intervention study is necessary because isolating a relevant independent variable with current dietary intervention protocols is impractical and impossible because it would require mass spectrometry testing of every possible food ingredient and compound in both legs of a study. This is due to:
- Extreme, and unknowable, variations of food contaminant concentrations from both Plastic-Derived Chemicals and Ultra-Processed Foods additives in most common food items.[139],[140],[141],[142],[143],[144],[145]
- Evidence of inherent contamination in production and processing and not solely from food contact materials.[146],[147],[148],[149],[150],[151]
- Ubiquitous contamination of all commonly available foods and the impractical need to use extreme procedures for sourcing food. See Appendix 2[152], and Appendix 3 in the IRB-approved study revision[153].
- Unknown co-founding interactions of micro-nutrients.[154]
This study will be a blinded human clinical trial with 24 subjects conducted in a strictly controlled dormitory environment. Investigators hypothesize that administering reagents Bisphenol A, Bisphenol F, and Bisphenol S to positive subjects in a clinically relevant daily dosage as defined by NHANES will increase inflammation and negatively affect metabolism. Because the United States FDA has not yet established a Tolerable Daily Intake for BPA, BPF, or BPS the study will adhere to the TDI set for BPA by the European Food Safety Authority (EFSA) at 4 µg/kg.[155] Blood samples of all subjects will be analyzed for hsCRP, TNF-alpha, and interleukin-6 (inflammation), and for metabolic indications: A1C, ghrelin (metabolic), and FGF-21. Testing will include cortisol testosterone, PSA (Prostate Specific Antigen) Blood samples will also be subject to mass spectrometry analysis for Bisphenol A, F & S. Urine samples will also be analyzed for Bisphenol A, F & S and the results compared with population data from NHANES. All subjects will consume the same menu every day. One week prior to the start of the trial, all subjects will be equipped with Apple Watches for baseline and continuous, recorded monitoring of sleep cycles, heart rate, and other vital signs which may be valuable in determining the effects of the dormitory setting. Subjects will be required to adhere to the same sleep and wake times as set by the trial venue. Substantial variance may result in disqualification from trial.
Subject Selection Criteria
Strict criteria will be enforced for matching trial subjects, as closely as possible to each other, and to “normal” in terms of health and other physical attributes and measurable norms such as percent body fat, metabolic health (blood panel), VO2max, and other factors. Selection criteria will include:
- Healthy adult males aged 21 to 35
- physical fitness,
- current diet,
- previous illnesses,
- personal microbiomes,
- current or previous legal or illegal drug use or abuse, (including alcohol and tobacco),
- current or previous chronic health conditions, prescription pharmaceutical use and,
- other unknowable factors that may affect epigenetic expression of the genome including those from life environment, socioeconomic status, and education.
- potential study subjects will not be accepted who require any prescription or non-prescription medicines during the trial. No NSAIDS within 48 hours of the beginning of the trial.
NOTE: Half of the test cohort was originally scheduled to be female. However, given that bisphenols are artificial estrogens, investigators realized that menstrual cycle hormone variations among female test subjects could create inconsistent data due to potential endocrinological variations and estrogenic influences. Those factors could possibly obscure or distort the effects of the bisphenols and make it problematic to draw valid conclusions in this small (24 subject) cohort. As an alternate way of including female subjects in the cohort, investigators considered accepting women who were taking prescription contraceptives, and who would be in the same timing phase (preferably week 2) when the trial began. However, upon further thought, investigators recognized that the different brands (and hormone composition) of available contraceptives may offer their own confounding endocrinological issues. That issue combined with how to determine whether all female subjects had adhered to their required daily consumption of pills, investigators reluctantly decided that the limited cohort should not include women as subjects in this trial of estrogen-mimicking chemicals at this time.
Screening
Potential investigation subjects will be exhaustively screened by a written survey, and those tentatively selected will be interviewed in person for the potential issues listed above. Subjects will also be evaluated for psychological and behavioral suitability to get along with strangers (other study subjects), within a structured regimen, and confined to a restricted dormitory environment for the length of the study. Subjects will be polled to determine food preferences in order to create a pleasing menu. Finally, prospective subjects will undertake a blood draw to determine if the study participant’s baseline serum concentrations are within appropriate limits. Food Food and other consumed substances will be treated as reagents and fundamental bench experiment scientific standards[156] will be observed for food acquisition, preparation, as modified for a previous human dietary intervention trial.[157] In addition, lessons learned from experience and previous trials will be observed.[158] In brief:
- All consumed substances (solid and liquid) will be measured to the nearest gram in both preparation and serving. Record keeping will be real-time using tablets such as iPads.
- Food preparation personnel will be trained for adherence to protocols and monitored for compliance. Pots, pans, utensils and all apparatus will be treated as laboratory equipment.
- Only glass and stainless steel would be allowed with limited use of wood when unavoidable. Carefully screened trial subjects should be sequestered in an appropriately conditioned and equipped human-centered dormitory environment to eliminate non-food exposures and other confounding environmental and stress-related psychological confounders.
- Other than properly filtered water, all subjects will consume the same amounts of food and drink and be required to consume all portions.
Bisphenol Dosing: A, F & S
All subjects will be required to consume all of a small serving of a familiar comestible fluid such as apple juice which will be served three times per day with meals. Only one of the servings to positive controls will be dosed on days 4, 5, and 6.
More details
Please see the sections on Reagents, and Dormitory and Subject Environment Protocols. The absence of causality and accurate reproducibility of human nutritional studies lie in their inability to accurately quantify relevant reagents, inadequately employed methodology, lack of adequate experimental oversight, test subject variability, inaccurate measurements, failure to avoid contamination, and inadequate environmental controls. This is further complicated because the human bio-chemosphere can react to compounds present in micro-, nano-, and pico- concentrations. To accurately claim causality in a dietary intervention study depends on providing the exact same foods, prepared the exact same way, served in exactly the same environmental conditions. Because there are many unknowable and co-confounding factors and compounds, exactitude requires maintaining all factors (every food, beverage, and non-food exposure) the same in the before and after legs of a trial, with the exception of a single compound that is dosed in the “contamination” leg. To assure the most accurate results, meals and beverages for both legs of the trial should be prepared as a single batch before the start of the trial and then be divided in half for each leg. After establishing an existing state (baseline reading), the first leg consists of half of the pre-prepared food and beverages that have not been dosed with the substance being evaluated. Following that, the second leg consists of the second half of the same food and beverages that have been dosed according to a recommended Daily Intake. That protocol changes only a single independent variable. As a result, the measured outcomes (hsCRP, Ghrelin, etc.) should therefore accurately warrant a valid conclusion that the change in the independent variable is causal.
Reagents
The most intractable issue is the imperative to describe “biological material with enough information to uniquely identify the reagents” (for example unique accession number in repository). Why foods fail as reagents In the case of a nutrition trial, every food must be treated as a reagent. However, edible foods have no complete census of chemical compounds. Why an “apple cannot be a reagent apple” Even basic foods such as an apple are subject to a myriad of variations including the species and cultivar of the tree from which it was harvested, harvest method (hand, machine), climate, soil composition, source of irrigation (natural, recycled municipal wastewater), fertilization (none, compost, animal manure, recycled municipal sludge), pesticide use, harvesting, packing, and transport details, cleansing (including method and source of water), the addition of a wax sealant (composition and application), handling, packing, and retail display (subject to water misting: In which case, composition of water, possible municipal or plastic contamination must also be ascertained). As a result, the closest that an investigator could possibly approach to having a reagent-qualified apple is to personally pick samples from the same farm then create a complete chemical profile of an apple closest to those to be served in a human trial. Replication, however, is unlikely because of the inability to obtain a sufficiently identical, reagent-grade apple. Complicating matters further is the inherent chemical complexity of foods, there are more than 10,000 ingredients legally allowed to be added to human food in the United States.[159] Disclosure of the use of those compounds is not legally required. Layered on top of this, there are more than 26,000 biochemicals in foods consumed by humans.[160] That study noted that only about 150 of those compounds are tracked by most food composition tables. Termed “nutritional dark matter,” the study noted that, “The absence of information on these untracked biochemicals could be responsible for inconsistencies in, and the irreproducibility of, published results as well as for missing health effects, and can also create spurious associations that are not replicable by meta-analysis.”
Off The Reagent Rails: Ultra-Processed Foods
Ultra-Processed foods (UPFs) inhabit a nebulous region of unknowable unknowns. The NOVA classification, originally proposed in 2010, has been widely adopted to define a range of food preparation categories.[161] A first attempt at codifying UPFs grew out of the 2016 United Nations Decade of Nutrition. ‘The term “ultra-processed” was coined to refer to industrial formulations manufactured from substances derived from foods or synthesized from other organic sources. They typically contain little or no whole foods, are ready-to-consume or heat up, and are fatty, salty or sugary and depleted in dietary fibre, protein, various micronutrients and other bioactive compounds. Examples include: sweet, fatty or salty packaged snack products, ice cream, sugar-sweetened beverages, chocolates, confectionery, French fries, burgers and hot dogs, and poultry and fish nuggets.’[162] That definition has undergone many efforts since them to better describe tragically adulterated foods high in salt, sugar, fats, manufactured substances, additives, preservatives — none of which has a publicly available list of complete ingredients. Significantly for replicability, there can be no guarantee that a given UPF will contain the same constituents in the same quantities from the same manufacturer. In that regard, a given package of “Yummy Gunk Snacks” containing unknown components will qualify as a definable reagent for the purpose of replication. This is further exacerbated by the fact that any observable outcome could be attributed to any given specific component. Despite that, irreplicable trials of UPFs continue as do attempts to define them.[163]
Further complications
- Extreme, and unknowable, variations of food contaminant concentrations from both Plastic-Derived Chemicals and Ultra-Processed Foods additives in most common food items.[164],[165],[166],[167],[168],[169],[170]
- Evidence of inherent contamination in production and processing and not solely from food contact materials.[171],[172],[173],[174],[175],[176],[177]
- Ubiquitous contamination of all commonly available foods and the impractical need to use extreme procedures for sourcing food. See Appendix 2[178], and Appendix 3 in the IRB-approved study revision[179].
- Unknown co-founding interactions of micro-nutrients[180]
Dosing is the only path
Because of the impossibility of adequately defining reagents in a study, the alternative is to dose a human diet with a safe, relevant exposure dose of the chemical studied. Among the controllable unknowns and confounding factors we seek to minimize by employing a sequestered dormitory venue in the testing environment are:
- Personal life variability including living, working and personal environment.
- Non-food contaminants.
The sequestered dormitory environment attempts to provide a standardized setting, free from outdoor air pollution, individual choices in foods, residential, occupational, educational, and recreational and other outdoor pollutants, viruses, microorganisms and other confounding factors in the large class of uncontrollable unknowns.
Requirements Cleaning
Polymers must be avoided at every opportunity. Microfiber towels and other items are strictly forbidden. Tables and chairs should be non-reactive, non-porous. To prevent the spread of microplastics, all polymer-based items — chairs, tables, cushions and other objects must be wiped down daily with a cotton cloth moistened by distilled water. This includes tables, chairs, work surfaces, exercise equipment, and electronic work and recreational devices brought in by test subjects, or provided by investigators. Floors will be cleaned daily with cotton-based mops and plain distilled water. Polymer-based materials are universally found in professional and institutional kitchens given to their durability and ease of cleaning. However, friction against the floor caused by shoes and rolling carts inevitably creates ultra-fine particles many of which qualify as PM2.5 which — regardless of chemical composition — cause inflammation when inhaled. Significantly, many of the polymers from which flooring is made (such as polyvinyl chloride) have known health effects and can contaminate food destined for test subjects. If a cleaning agent is required, it should be hypochlorite-based and free of surfactants such as ethoxylates that have been shown to cause health effects. Bed mattresses will be cleaned and sanitized as per hospital regulations. Sheets will be 100-percent cotton. Bottom sheets will be high-thread count (500, single-ply) and doubled up to prevent potential migration of any remaining microplastics or other particles on the mattresses. The same cleaning agents and protocols should be employed. No paper towels. Rooms will be identically equipped and all surfaces cleaned with an acceptable detergent and implements. If dormitory flooring is vinyl or polymer based, all chair legs will be covered with a sock-like cotton covering or fabric pad to reduce microplastic contamination. No chairs with rolling wheels will be permitted.
Environment – Floor, walls, ventilation PM2.5
Before the facility is prepared and provisioned, the dormitory area must cleared of all prohibited items and thoroughly cleaned. Afterwards, the HVAC filter should be changed and the system fan run continuously for at least four hours to collect as much dust as possible which has been stirred up from cleaning. A fresh filter should then be installed. Constant ventilation of HEPA-filtered air is vital to prevent contamination with polymer-based particles. The PM 2.5 is implicated in a wide range of serious health effects[181],[182],[183] and is most likely to reach and be retained by alveoli and thus have direct access to the bloodstream. As a consequence, air will be constantly monitored throughout the trial and records kept. Purple Air sensors will be installed in every room. PurpleAir[184] is an accurate and cost-effective[185] solution for monitoring. Other than the cost of the sensor ($179), monitoring is free and historical data can be downloaded and saved. All rooms will be equipped with a HEPA PM2.5-capable air filter device which also provides ultraviolent light, and micro-organism-protective features. This filter device should also provide activated charcoal filtration for adsorption of organic compounds. These can play a role in removing small organic compounds that can potentially affect study biomarkers. Some of those same odors may also produce objectionable odors. Small rooms will use a filter such as the GermGuardian. Larger rooms will require a higher capacity device. The type and age of the HVAC system should be noted as well as the type of filter used and the ducting materials (metal versus plastic), along with make, model, capacity, and type (portable or installed). Locations of permanent vents and air returns should be noted and diagrammed. Locations of portable units must be noted along with the distance from the HVAC air return. All filters must be newly installed. Towels, Napkins, and All Other
Cloth Material – Sourcing and Washing
Towels, napkins and all other cloth material will be 100% cotton. All cotton fabric will be sourced from that which is grown organically, without pesticides. All items will be laboratory tested for pesticide and plastic residues. The source should be noted in the list of materials. Before first use, study towels should be washed and dried twice to remove any potential contaminants.
Bedding
Bedding will be 100% cotton. Multiple layers will be installed to minimize migration of plastic from sanitary mattress covers which are expected to block migration from mattress materials.
Towel, apron and other cloth washing
A new washer and dryer should be a purchased because difficult-to-remove fabric softener and detergent residues can build up on the internal surfaces up from phthalates and other chemicals contained in detergents and fabric softeners. These can be basic, no-frills consumer-level, budget units.
Clothing
Subjects will be provided with a choice of all-cotton under-garments (possibly scrubs). All-cotton outerwear will be provided. All garments will be pre-washed multiple (TBD) times using approved detergents (no rinses or other additives) to reduce/remove sizing or possible plastics contamination. All garments will be changed daily. Study-issued socks and slippers will be issued. Exercise shoes and cotton socks will be allowed in the ventilated exercise area only. Shoes must not be worn outside that area unless covered in cotton booties.
Personal Products and Considerations
Soap and shampoo with minimum potential for aerosolization of objectionable compounds will be provided. Daily showers will be allowed but investigators must determine appropriate ventilation to prevent spread of potentially unknown plastic chemicals and nanoparticles. Opportunities for exercise must be investigated, but remain problematic because of the use of plastics in exercise equipment. This may be possible by the use of proper ventilation to remove plastic contamination from leaching and frictional shedding of micro/nanoplastics. Psychological factors must be considered and resolved including need for privacy, minimum stress (to reduce cortisol levels), possible conflicts among study participants. This will be particularly important for sleeping arrangements unless it’s possible to provide a private room for each participant. Creative partitioning in multiple-occupant rooms might provide a solution.
Personal Product Contaminants
Sathyanarayana et al (2013)[186] posited that substantial non-food exposures such as those with personal care products could mask BPA and phthalate reductions from dietary intervention. However, a quick picture of the complexity of NFEs can be illustrated by this diagram from Dodson et al (2012)[187]: An extensive discussion of NFE contamination sources may be accessed in Appendix 4.
Triaging NFE elimination
Given the impossibility of avoiding BPA, phthalates and other harmful chemicals in the environment, reproducibility of studies must focus on the practical reduction of known exposures reducing those exposures that are:
- most easily identified and,
- practical to control,
- likely to produce maximum reduction via minimum study subject effort, and
- can be adequately documented and replicated.
As a potential starting point for assessing personal environmental exposures, investigators will consider the EDC Footprint calculator[188] developed by the Pennsylvania State University Extension Service. While the Excel version[189] was designed to offer an indication of contamination products found in municipal wastewater and is not all-inclusive, it could be modified and updated to better serve the purposes of this study, or future efforts. Personal environment controls 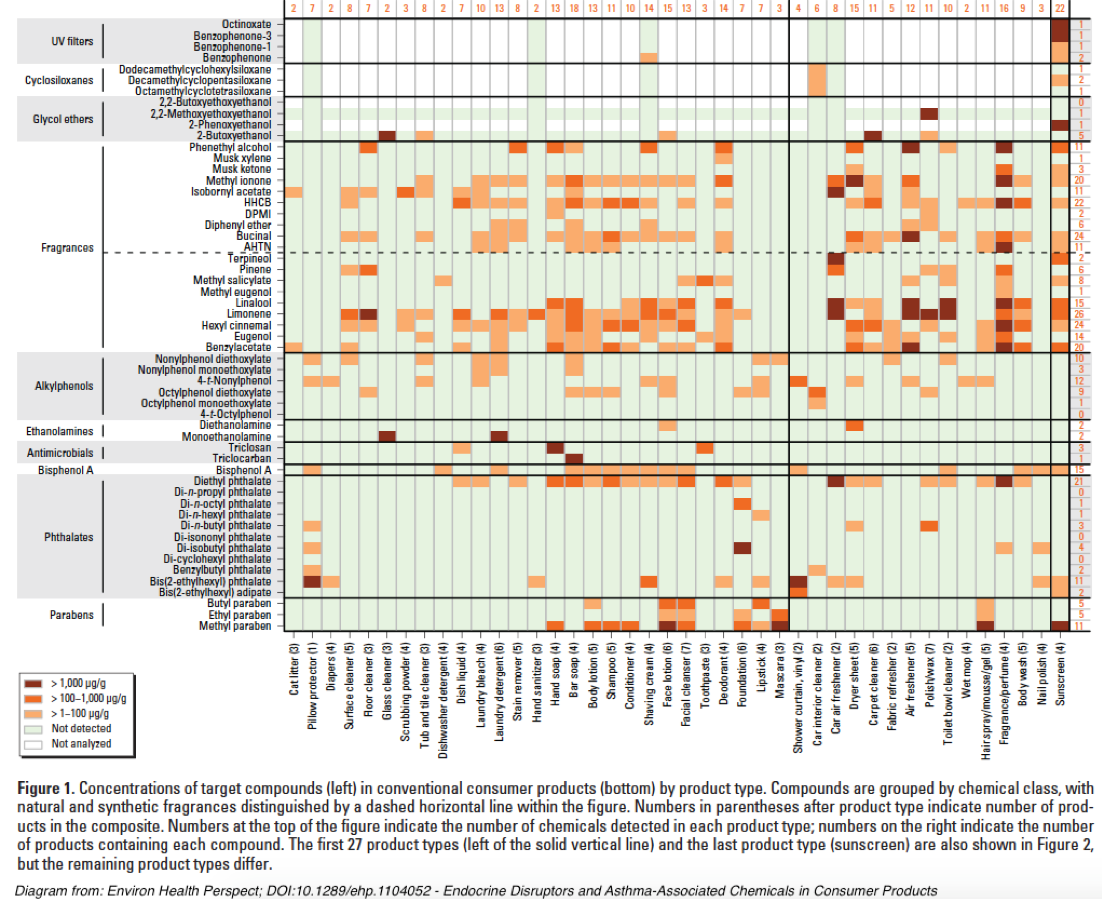 As the above chart from Dodson et al (2012) illustrates, most personal care products contain relevant chemical contaminants that can potentially confound this study’s chosen health effects markers.
As the above chart from Dodson et al (2012) illustrates, most personal care products contain relevant chemical contaminants that can potentially confound this study’s chosen health effects markers.
- Recommended toothpaste without Triclosan and reduced or zero-levels of contaminants.
- Uncoated nylon fiber dental floss (no plastic strips).
- No hand creams or lotions.
- Use recommended shampoo with reduced or zero-levels of contaminants.
- Use recommended hand soap with reduced or zero-levels of contaminants.
- Use recommended deodorant.
- No contact lenses. Use of eyeglasses only.
- No contact with cash register receipts.
- No hair spray, gel or other treatment.
[1] Perdue, W.L., Reus, V.I., van Breemen, R.B. Munchiri, R.N., and Yeamans-Irwin, R.L. 2022. Stricter protocols combined with a clinical serum biomarker can increase replicability and causality for dietary intervention studies. Plus empirical data on BPA regrettable substitutions. medRxi: doi: https://doi.org/10.1101/2022.08.09.22278588
[2] Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metabolism. 2019;30(1):67–77.e3. doi:10.1016/j.cmet.2019.05.008
[3] Perdue, L. (n.d.). Accounting for Unknown Interactions of Co-existing Factors that Confounded Causality Conclusions in Two Studies | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1661
[4] Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Cien Saude Colet. 2012;17(2):407–434. doi:10.1590/S1413-81232012000200015
[5] United States Environmental Protection Agency (US EPA). TSCA Chemical Substance Inventory. US EPA. Published August 15, 2014. Accessed August 31, 2020. https://www.epa.gov/tsca-inventory
[6] European Food Safety Authority (EFSA). Report of ESCO WG on non plastic FCMs. EFSA Supporting Publications. 2012;8(7):139E. doi:10.2903/sp.efsa.2011.EN-139
[7] Neltner TG, Kulkarni NR, Alger HM, et al. Navigating the U.S. Food Additive Regulatory Program. Compr Rev Food Sci Food Saf. 2011;10:342–368. doi:10.1111/j.1541-4337.2011.00166.x
[8] Muncke J, Myers JP, Scheringer M, Porta M. Food packaging and migration of food contact materials: will epidemiologists rise to the neotoxic challenge? J Epidemiol Community Health. 2014;68(7):592–594. doi:10.1136/jech-2013-202593
[9] Centers for Disease Control and Prevention (CDC). Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. Accessed August 31, 2020. https://www.cdc.gov/exposurereport/index.html
[10] Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177. doi:10.1016/j.reprotox.2007.07.010
[11] Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35:S5–S24. doi:10.1016/j.semcancer.2015.03.005
[12] Keri RA, Ho S-M, Hunt PA, Knudsen KE, Soto AM, Prins GS. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol. 2007;24(2):240–252. doi:10.1016/j.reprotox.2007.06.008
[13] Fang L, Wuptra K, Chen D, et al. Environmental-stress-induced Chromatin Regulation and its Heritability. J Carcinog Mutagen. 2014;5(1). doi:10.4172/2157-2518.1000156
[14] Vega A, Baptissart M, Caira F, Brugnon F, Lobaccaro J-MA, Volle DH. Epigenetic: a molecular link between testicular cancer and environmental exposures. Front Endocrinol. 2012;3. doi:10.3389/fendo.2012.00150
[15] Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho S-M. Exposure to Bisphenol A Correlates with Early-Onset Prostate Cancer and Promotes Centrosome Amplification and Anchorage-Independent Growth In Vitro. PLoS One. 2014;9(3):e90332. doi:10.1371/journal.pone.0090332
[16] Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N., Welshons, W.V. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24: 139–177
[17] Zhang Z, Chen S, Feng Z, Su LJ. Pregnancy Exposures Determine Risk of Breast Cancer in Multiple Generations of Offspring. In: Su LJ, Chiang T, eds. Environmental Epigenetics. Molecular and Integrative Toxicology. Springer; 2015:75–103. doi:10.1007/978-1-4471-6678-8_5
[18] Gassman NR, Coskun E, Stefanick DF, et al. Bisphenol A Promotes Cell Survival Following Oxidative DNA Damage in Mouse Fibroblasts. PLoS One. 2015;10(2):e0118819. doi:10.1371/journal.pone.0118819
[19] Bishop KS, Ferguson LR. The Interaction between Epigenetics, Nutrition and the Development of Cancer. Nutrients. 2015;7(2):922–947. doi:10.3390/nu7020922
[20] Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12(1):1–9. doi:10.7497/j.issn.2095-3941.2015.0006
[21] Gao X, Wang H-S. Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms. Int J Environ Res Public Health. 2014;11(8):8399–8413. doi:10.3390/ijerph110808399
[22] Rancière F, Lyons JG, Loh VHY, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environmental Health. 2015;14(1):46. doi:10.1186/s12940-015-0036-5
[23] Bae S, Hong Y-C. Exposure to Bisphenol A From Drinking Canned Beverages Increases Blood Pressure. Hypertension. 2015;65(2):313–319. doi:10.1161/HYPERTENSIONAHA.114.04261
[24] Belcher SM, Chen Y, Yan S, Wang H-S. Rapid Estrogen Receptor-Mediated Mechanisms Determine the Sexually Dimorphic Sensitivity of Ventricular Myocytes to 17β-Estradiol and the Environmental Endocrine Disruptor Bisphenol A. Endocrinology. 2012;153(2):712–720. doi:10.1210/en.2011-1772
[25] Gao X, Liang Q, Chen Y, Wang H-S. Molecular Mechanisms Underlying the Rapid Arrhythmogenic Action of Bisphenol A in Female Rat Hearts. Endocrinology. 2013;154(12):4607–4617. doi:10.1210/en.2013-1737
[26] Liang Q, Gao X, Chen Y, Hong K, Wang H-S. Cellular Mechanism of the Nonmonotonic Dose Response of Bisphenol A in Rat Cardiac Myocytes. Environ Health Perspect. 2014;122(6):601–608. doi:10.1289/ehp.1307491
[27] Melzer D, Osborne NJ, Henley WE, et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation. 2012;125(12):1482–1490. doi:10.1161/CIRCULATIONAHA.111.069153
[28] Yan S, Song W, Chen Y, Hong K, Rubinstein J, Wang H-S. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia–reperfusion in female rat hearts. Food Chem Toxicol. 2013;56:75–80. doi:10.1016/j.fct.2013.02.011
[29] Regnier SM, Sargis RM. Adipocytes under assault: Environmental disruption of adipose physiology. Biochim Biophys Acta Mol Basis Dis. 2014;1842(3):520–533. doi:10.1016/j.bbadis.2013.05.028
[30] Ellero-Simatos S, Claus SP, Benelli C, et al. Combined Ellero-Simatos S, Claus SP, Benelli C, et al. Combined Transcriptomic–1H NMR Metabonomic Study Reveals That Monoethylhexyl Phthalate Stimulates Adipogenesis and Glyceroneogenesis in Human Adipocytes. J Proteome Res. 2011;10(12):5493–5502. doi:10.1021/pr200765v
[31] Marmugi A, Ducheix S, Lasserre F, et al. Low doses of bisphenol a induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55(2):395–407. doi:10.1002/hep.24685
[32] Hugo Eric R., Brandebourg Terry D., Woo Jessica G., Loftus Jean, Alexander J. Wesley, Ben-Jonathan Nira. Bisphenol A at Environmentally Relevant Doses Inhibits Adiponectin Release from Human Adipose Tissue Explants and Adipocytes. Environ Health Perspect. 2008;116(12):1642–1647. doi:10.1289/ehp.11537
[33] Menale C, Piccolo MT, Cirillo G, et al. Bisphenol A effects on gene expression in adipocytes from children: association with metabolic disorders. J Mol Endocrinol. 2015;54(3):289–303. doi:10.1530/JME-14-0282
[34] Savastano S, Tarantino G, D’Esposito V, et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med. 2015;13(1):169. doi:10.1186/s12967-015-0532-y
[35] Seidlová-Wuttke D, Jarry H, Christoffel J, Rimoldi G, Wuttke W. Effects of bisphenol-A (BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: A 3 months comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology. 2005;213(1):13–24. doi:10.1016/j.tox.2005.05.001
[36] Alonso-Magdalena Paloma, Morimoto Sumiko, Ripoll Cristina, Fuentes Esther, Nadal Angel. The Estrogenic Effect of Bisphenol A Disrupts Pancreatic β-Cell Function In Vivo and Induces Insulin Resistance. Environ Health Perspect. 2006;114(1):106–112. doi:10.1289/ehp.8451
[37] Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1):63–68. doi:10.1016/j.mce.2009.02.016
[38] Bouchard L, Thibault S, Guay S-P, et al. Leptin Gene Epigenetic Adaptation to Impaired Glucose Metabolism During Pregnancy. Diabetes Care. 2010;33(11):2436–2441. doi:10.2337/dc10-1024
[39] Hofmann PJ, Schomburg L, Köhrle J. Interference of Endocrine Disrupters with Thyroid Hormone Receptor–Dependent Transactivation. Toxicol Sci. 2009;110(1):125–137. doi:10.1093/toxsci/kfp086
[40] Schmutzler C, Bacinski A, Gotthardt I, et al. The Ultraviolet Filter Benzophenone 2 Interferes with the Thyroid Hormone Axis in Rats and Is a Potent in Vitro Inhibitor of Human Recombinant Thyroid Peroxidase. Endocrinology. 2007;148(6):2835–2844. doi:10.1210/en.2006-1280
[41] Patisaul HB. Achieving CLARITY on bisphenol A, brain and behaviour. J Neuroendocrinol. 2020;32(1):e12730. doi:10.1111/jne.12730
[42] Wiersielis KR, Samuels BA, Roepke TA. Perinatal exposure to bisphenol A at the intersection of stress, anxiety, and depression. Neurotoxicol Teratol. 2020;79:106884. doi:10.1016/j.ntt.2020.106884
[43] Fang F, Chen D, Yu P, et al. Effects of Bisphenol A on glucose homeostasis and brain insulin signaling pathways in male mice. Gen Comp Endocrinol. 2015;212:44–50. doi:10.1016/j.ygcen.2015.01.017
[44] El-Missiry MA, Othman AI, Al-Abdan MA, El-Sayed AA. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J Neurol Sci. 2014;347(1):251–256. doi:10.1016/j.jns.2014.10.009
[45] Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–1093. doi:10.1016/j.bbi.2011.02.005
[46] Testa C, Nuti F, Hayek J, et al. Di-(2-Ethylhexyl) Phthalate and Autism Spectrum Disorders: ASN Neuro. Published online May 30, 2012. doi:10.1042/AN20120015
[47] Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial β-oxidation by long chain acyl-CoA dehydrogenase. Medical Hypotheses. 2004;62(6):970–975. doi:10.1016/j.mehy.2004.01.011
[48] Hannon PR, Peretz J, Flaws JA. Daily Exposure to Di(2-ethylhexyl) Phthalate Alters Estrous Cyclicity and Accelerates Primordial Follicle Recruitment Potentially Via Dysregulation of the Phosphatidylinositol 3-Kinase Signaling Pathway in Adult Mice. Biol Reprod. 2014;90(6). doi:10.1095/biolreprod.114.119032
[49] Hannon PR, Flaws JA. The Effects of Phthalates on the Ovary. Front Endocrinol. 2015;6. doi:10.3389/fendo.2015.00008
[50] León-Olea M, Martyniuk CJ, Orlando EF, et al. Current Concepts in Neuroendocrine Disruption. Gen Comp Endocrinol. 2014;0:158–173. doi:10.1016/j.ygcen.2014.02.005
[51] Meeker JD, Ferguson KK. Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012. J Clin Endocrinol Metab. 2014;99(11):4346–4352. doi:10.1210/jc.2014-2555
[52] Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–466. doi:10.1038/jes.2013.69
[53] Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int. 2008;34(7):1033–1049. doi:10.1016/j.envint.2008.01.004
[54] Lyche JL, Gutleb AC, Bergman Å, et al. Reproductive and Developmental Toxicity of Phthalates. J Environ Sci Health B. 2009;12(4):225–249. doi:10.1080/10937400903094091
[55] Wetherill YB, Akingbemi BT, Kanno J, et al. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology. 2007;24(2):178–198. doi:10.1016/j.reprotox.2007.05.010
[56] Resendiz M, Mason S, Lo C-L, Zhou FC. Epigenetic regulation of the neural transcriptome and alcohol interference during development. Front Genet. 2014;5. doi:10.3389/fgene.2014.00285
[57] Mason S, Zhou FC. Editorial: Genetics and epigenetics of fetal alcohol spectrum disorders. Front Genet. 2015;6. doi:10.3389/fgene.2015.00146
[58] Kim JH, Sartor MA, Rozek LS, et al. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15(1):30. doi:10.1186/1471-2164-15-30
[59] Walker CL. Epigenomic reprogramming of the developing reproductive tract and disease susceptibility in adulthood. Teratology. 2011;91(8):666–671. doi:10.1002/bdra.20827
[60] Cao J, Rebuli ME, Rogers J, et al. Prenatal Bisphenol A Exposure Alters Sex-Specific Estrogen Receptor Expression in the Neonatal Rat Hypothalamus and Amygdala. Toxicol Sci. 2013;133(1):157–173. doi:10.1093/toxsci/kft035
[61] Crinnion WJ. Toxic Effects of the Easily Avoidable Phthalates and Parabens. Environmental Medicine. Published online 2010:7.
[62] Wang I-J, Karmaus WJ, Chen S-L, Holloway JW, Ewart S. Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clinical Epigenetics. 2015;7(1):27. doi:10.1186/s13148-015-0060-x
[63] Dodson Robin E., Nishioka Marcia, Standley Laurel J., Perovich Laura J., Brody Julia Green, Rudel Ruthann A. Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ Health Perspect. 2012;120(7):935–943. doi:10.1289/ehp.1104052
[64] Hoppin Jane A., Jaramillo Renee, London Stephanie J., et al. Phthalate Exposure and Allergy in the U.S. Population: Results from NHANES 2005–2006. Environ Health Perspect. 2013;121(10):1129–1134. doi:10.1289/ehp.1206211
[65] Wang, I.-J., Karmaus, W. J., Chen, S.-L., Holloway, J. W., and Ewart, S. 2015. Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clinical Epigenetics 7(1): 27.
[66] Dodson, R.E., Nishioka, M., Standley, L.J., Perovich, L.J., Brody, J.G., and Rudel, R.A. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental Health Perspectives 120(7): 935.
[67] Hoppin, J.A., Jaramillo, R., London, S.J., Bertelsen, R.J., Salo, P.M., Sandler, D.P., Zeldin, D.C. 2013. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005–2006. Environmental Health Perspectives 121: 1129–1134.
[68] vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research. 2006;100(1):50–76. doi:10.1016/j.envres.2005.09.001
[69] Baldi E, Muratori M, eds. Genetic Damage in Human Spermatozoa. 1st ed. Springer; 2014. doi:10.1007/978-1-4614-7783-9
[70] Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293–342. doi:10.1210/er.2009-0002
[71] Bao W, Liu B, Rong S, Dai SY, Trasande L, Lehmler H-J. Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw Open. 2020;3(8):e2011620–e2011620. doi:10.1001/jamanetworkopen.2020.11620
[72] Perdue, W.L., Reus, V.I., van Breemen, R.B. Munchiri, R.N., and Yeamans-Irwin, R.L. 2022. Stricter protocols combined with a clinical serum biomarker can increase replicability and causality for dietary intervention studies. Plus empirical data on BPA regrettable substitutions. medRxi: doi: https://doi.org/10.1101/2022.08.09.22278588
[73] Winkler, J., Liu, P., Phong, K., and Z. Werb. 2022. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammory gland organoid morphology and proteome. PNAS Cell Biology 119(11): e2115308119
[74] Ji, G., Gu, J., Guo, M., Zhou, L., Wang, Z., Shi, L., and A. Gu. 2022. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere 291(2): 132936.
[75] Kim, J.I., Lee, Y.A., Shin, C.H., Hong, Y-C., Kim, B-N., and Y-H. Lim. 2022. Association of bisphenol A, bisphenol F, and bisphenol S with ADHD symptoms in children. Environment International 161: 107093.
[76] Joseph Apau, Akwasi Acheampong & Eric Adua | Bryan M. Wong (Reviewing editor) (2018) Exposure to bisphenol A, bisphenol F, and bisphenol S can result in obesity in human body, Cogent Chemistry, 4:1, DOI: 10.1080/23312009.2018.1506601
[77] Soria Eladak, Tiphany Grisin, Delphine Moison, Marie-Justine Guerquin, Thierry N’Tumba-Byn, Stéphanie Pozzi-Gaudin, Alexandra Benachi, Gabriel Livera, Virginie Rouiller-Fabre, René Habert, A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound, Fertility and Sterility, Volume 103, Issue 1, 2015, Pages 11–21
[78] Liu B, Lehmler HJ, Sun Y, Xu G, Sun Q, Snetselaar LG, Wallace RB, Bao W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab J. 2019 Feb;43(1):59–75. doi: 10.4093/dmj.2018.0045. PMID: 30793552; PMCID: PMC6387872.
[79] Liu B, Lehmler HJ, Sun Y, Xu G, Sun Q, Snetselaar LG, Wallace RB, Bao W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab J. 2019 Feb;43(1):59–75. doi: 10.4093/dmj.2018.0045. PMID: 30793552; PMCID: PMC6387872.
[80] Qadeer, Abdul, Kelly L. Kirsten, Zeeshan Ajmal, Xia Jiang, and Xingru Zhao. Alternative Plasticizers As Emerging Global Environmental and Health Threat: Another Regrettable Substitution?. Environmental science & technology (2022).
[81] Winkler, Juliane, Pengyuan Liu, Kiet Phong, Johanna H. Hinrichs, Nassim Ataii, Katherine Williams, Elin Hadler-Olsen et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome. Proceedings of the National Academy of Sciences 119, no. 11 (2022): e2115308119.
[82] Zuowei Ji, Jie Liu, Sugunadevi Sakkiah, Wenjing Guo, and Huixiao Hong ACS Sustainable Chemistry & Engineering 2021 9 (6), 2433–2446 DOI: 10.1021/acssuschemeng.0c09276
[83] Da Chen, Kurunthachalam Kannan, Hongli Tan, Zhengui Zheng, Yong-Lai Feng, Yan Wu, and Margaret Widelka Environmental Science & Technology 2016 50 (11), 5438–5453 DOI: 10.1021/acs.est.5b05387
[84] Huang M, Liu S, Fu L, Jiang X, Yang M. Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere. 2020 Aug;253:126707. doi: 10.1016/j.chemosphere.2020.126707. Epub 2020 Apr 9. PMID: 32289607. [85] Shamhari A’, Abd Hamid Z, Budin SB, Shamsudin NJ, Taib IS. Bisphenol A and Its Analogues Deteriorate the Hormones Physiological Function of the Male Reproductive System: A Mini-Review. Biomedicines. 2021 Nov 22;9(11):1744. doi: 10.3390/biomedicines9111744. PMID: 34829973; PMCID: PMC8615890. [86] Andújar N, Gálvez-Ontiveros Y, Zafra-Gómez A, Rodrigo L, Álvarez-Cubero MJ, Aguilera M, Monteagudo C, Rivas A. Bisphenol A Analogues in Food and Their Hormonal and Obesogenic Effects: A Review. Nutrients. 2019; 11(9):2136. https://doi.org/10.3390/nu11092136
[87] Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866.
[88] Kojima, H.; Takeuchi, S.; Sanoh, S.; Okuda, K.; Kitamura, S.; Uramaru, N.; Sugihara, K.; Yoshinari, K. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 2019, 413, 48–55.
[89]Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.H.; Svoboda, D.; Auerbach, S.S.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235.
[90] Punt, A.; Aartse, A.; Bovee, T.F.H.; Gerssen, A.; Van Leeuwen, S.P.J. Hoogenboom RLAP, Peijnenburg AACM. Quantitative in vitro-to-in vivo extrapolation (QIVIVE) of estrogenic and anti-androgenic potencies of BPA and BADGE analogues. Arch. Toxicol. 2019, 93, 1941–1953.
[91] Carra, R.J. 2011. It’s in Our Blood: A Critique of the FDA’s Reluctance to Regulate the Use of Bisphenol A in the Food Supply. Journal of Healthcare Law and Policy 14 (1).
[92] Freedman, L.P., Cockburn, I.M., and T.S. Simcoe. 2015. The economics of reproducibility in preclinical research. PLOS Biology; https://doi.org/10.1371/journal.pbio.1002165.
[93] Strauss, M. (2020, May 30). Americans are divided over the use of animals in scientific research. Pew Research Center. https://www.pewresearch.org/fact-tank/2018/08/16/americans-are-divided-over-the-use-of-animals-in-scientific-research/
[94] Baker, M. 2016. 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454. https://doi.org/10.1038/533452a
[95] Drucker, D.J. 2016. Never waste a good crisis: Confronting reproducibility in translational research. Cell Metabolism 24(3): 348-360.
[96] Vom Saal, Frederick S. Flaws in design, execution and interpretation limit CLARITY‐BPA’s value for risk assessments of bisphenol A. Basic & Clinical Pharmacology & Toxicology 125 (2019): 32-43.
[97] Vandenberg LN, Hunt PA, and AC Gore. 2019. Endocrine disruptors and the future of toxicology testing – lessons from CLARITY-BPA. Nature Reviews: Endocrinology 15(6):366‐374. doi:10.1038/s41574-019-0173-y
[98] Gerona, R., Vom Saal, F.S., and P.A. Hunt. 2020. BPA: have flawed analytical techniques compromised risk assessments?. The Lancet Diabetes & Endocrinology 8.1: 11-13.
[99]Vandenberg, L.N., Prins, G.S., Patisaul, H.B., and R.T. Zoelle. 2020. The Use and Misuse of Historical Controls in Regulatory Toxicology: Lessons from the CLARITY-BPA Study. Endocrinology 161(5): bqz014. https://doi.org/10.1210/endocr/bqz014
[100] RR-9: CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats. National Toxicology Program, U.S. Department of Health and Human Services. https://tools.niehs.nih.gov//cebs3/views/?action=main.dataReview&bin_id=3856 Accessed 3/29/2023. [101] Myers, J.P., Vom Saal, F.S., Akingbemi, B.T., Arizono, K., Belcher, S., Colborn, T., Chahoud, I., Crain, D.A., Farabollini, F., Guilette Jr., L.J., Hassold, T., Ho, S-M., Hunt, P.A., Iguchi, T., Jobling, S, Kanno, J., Laufer, H., Marcus, M., McLachlan, J.A., Nadal, A., Oehlmann, J., Olea, N., Palanza, P., Parmigiani, S., Rubin, B.S., Schoenfelder, G., Sonnenchein, C., Soto, A.M., Talsness, C.E., Taylor, J.A., Vandenberg, L.N., Vandenbergh, J.G., Vogel, S., Watson, C.S., Welchons, W.V., and R.T. Zoeller. 2009. Why public health agencies cannot depend on Good Laboratory Practices as a criterion for selecting data: The case of bisphenol A. Environmental Health Perspectives 117(3): DOI 10.1289/ehp.0800173 [102] Baldeshwiler, A. 2003. History of FDA Good Laboratory Practices. The Quality Assurance Journal 7(3): 153-228. [103] Abbott, A. 1999. Science comes to terms with lessons of fraud. Nature 398: 13-14. DOI https://doi.org/10.1038/17883 [104] FDA Grand Rounds, “Bisphenol A: Toxicology and Pharmacokinetic Data to Inform On-Going Safety Assessments,” presented by K. Barry Delclos, Ph.D., NCTR, 9/13/2018, 12-1pm EST. (n.d.). https://collaborationcqpub1.fda.gov/content/connect/c1/7/en/events/event/shared/default_template/event_landing.html?sco-id=145506454 [105] Society, E. (2019, November 15). Endocrine Society experts express concern with FDA statement on BPA safety. Endocrine Society. https://www.endocrine.org/news-and-advocacy/news-room/2018/endocrine-society-experts-express-concern-with-fda-statement-on-bpa-safety [106] Prins, G.S., Patisaul, H.B., Belcher, S.M., and L.N. Vandenberg. 2019. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic & Clinical Pharmacology & Toxicology 125(Suppl3): 14-31. [107] Akhtar, A. 2015. The flaws and human harms of animal experimentation. Cambridge Quarterly of Healthcare Ethics 24(4): 407-419. [108] Patil, P., Peng, R.D. & J.T. Leek. 2019. A visual tool for defining reproducibility and replicability. Nature Human Behavior 3: 650–652. https://doi.org/10.1038/s41562-019-0629-z [109] Goodman, S.N., Fanelli, D., and J.P.A. Ionnidis. 2016. What does research reproducibility mean? Science Translational Medicine 8(341). DOI: 10.1126/scitranslmed.aaf502 [110] Begley, C.G., and J.P.A. Ionnidis. 2015. Reproducibility in science: Improving the standard for basic and preclinical research. Circulation Research 116(1): 116-126. https://doi.org/10.1161/CIRCRESAHA.114.303819 [111] A., & Schwartz, L. (2022, December 1). 5 Foods We Thought Were Bad For Us, Now Turn Out to Be Good. Alternet.org. https://www.alternet.org/2015/04/5-foods-we-thought-were-bad-us-now-turn-out-be-good [112] Guasch-Ferré M, Bulló M, Martínez-González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M, Lapetra J, Vinyoles E, Lamuela-Raventós RM, Serra-Majem L, Pintó X, Ruiz-Gutiérrez V, Basora J, Salas-Salvadó J; PREDIMED study group. 2013. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Medicine. 3 Jul 16;11:164. doi: 10.1186/1741-7015-11-164. PMID: 23866098; PMCID: PMC3738153. [113] Rohrmann, S., Faeh, D. 2013. Should we go nuts about nuts?. BMC Medicine 11 (165). https://doi.org/10.1186/1741-7015-11-165 [114] Cardona-Morrell, M., Rychetnik, L., Morrell, S.L., Espinel, P.T., and A. Bauman. 2010. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 10 (653). https://doi.org/10.1186/1471-2458-10-653 [115] Garza C, Stover PJ, Ohlhorst SD, Field MS, Steinbrook R, Rowe S, Woteki C, and E. Campbell. 2019. Best practices in nutrition science to earn and keep the public’s trust. American Journal of Clinical Nutrition Jan 1;109(1):225-243. doi: 10.1093/ajcn/nqy337. PMID: 30657846; PMCID: PMC6900562. [116] Flier JS. Irreproducibility of published bioscience research: Diagnosis, pathogenesis and therapy. 2016. Molecular Metabolism. Nov 21;6(1):2-9. doi: 10.1016/j.molmet.2016.11.006. PMID: 28123930; PMCID: PMC5220388. [117] Akhtar, A. 2015. The flaws and human harms of animal experimentation. Cambridge Quarterly of Healthcare Ethics 24(4): 407-419. [118] National Human Genome Institute. (2023, March 3). Mouse Model. https://www.genome.gov/genetics-glossary/Mouse-Model [119] National Human Genome Institute. (2010, July 23). Why Mouse Matters. https://www.genome.gov/10001345/importance-of-mouse-genome [120] Drucker, D.J. 2016. Never waste a good crisis: Confronting reproducibility in translational research. Cell Metabolism 24(3): 348-360. [121] Resnik, D.B. 2011. Scientific research and the public trust. Science and Engineering Ethics 17(3): 399-409. [122] Akhtar, A. 2015. The flaws and human harms of animal experimentation. Cambridge Quarterly of Healthcare Ethics 24(4): 407-419. [123] National Human Genome Institute. (2023, March 3). Mouse Model. https://www.genome.gov/genetics-glossary/Mouse-Model [124] National Human Genome Institute. (2010, July 23). Why Mouse Matters. https://www.genome.gov/10001345/importance-of-mouse-genome [125] Drucker, D.J. 2016. Never waste a good crisis: Confronting reproducibility in translational research. Cell Metabolism 24(3): 348-360. [126] Moreno JD, Schmidt U, & S. Joffe. 2017. The Nuremberg Code 70 Years Later. JAMA 318(9):795–796. doi:10.1001/jama.2017.10265 [127] Rothman, David. (2001). Human Experimentation and Research. Routledge. eBook ISBN9781315198231 [128] George J. Annas. 2018. Beyond Nazi War Crimes Experiments: The Voluntary Consent Requirement of the Nuremberg Code at 70. American Journal of Public Health 108(1): 42-46. [129] Evelyne Shuster. 2018. American Doctors at the Nuremberg Medical Trial. American Journal of Public Health 108(1): 47-52. [130] Calafat AM, Ye X, Wong LY, Reidy JA, & LL Needham. 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental Health Perspectives 116(1):39-44. doi: 10.1289/ehp.10753. [131] National Health and Nutrition Examination Survey (NHANES) DNA Specimens: Guidelines for Proposals To Use Samples and Cost Schedule. (2019, February 21). Federal Register. https://www.federalregister.gov/documents/2019/02/21/2019-02908/national-health-and-nutrition-examination-survey-nhanes-dna-specimens-guidelines-for-proposals-to [132] NHANES – Informed Consent. (n.d.). https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm [133] Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, Whyatt RM, and MS Wolff. 2015. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environmental Health Perspectives 123(7):A166-8. doi: 10.1289/ehp.1510041 [134] Ryan J. Carra. 2011. It’s in Our Blood: A Critique of the FDA’s Reluctance to Regulate the Use of Bisphenol A in the Food Supply. Journal of Health Care Law and Policy 14: 153 [135] NHANES – NCHS Research Ethics Review Board Approval. (n.d.). https://www.cdc.gov/nchs/nhanes/irba98.htm [136] Perdue, L. (2014, July 3). Original Sources: 84,000 Legal Chemicals. Fewer Than 200 Tested. Only 5 Ever Banned. Here’s Where Those Numbers Come From | The Stealth Syndromes Project. http://stealthsyndromes.com/?p=1026 [137] Thayer, K.A., Doerge, D.R., Hunt, D., Schurman, S.H., Twaddle, N.C., Churchwell, M.I., Garantziotis, S., Kissling, G.E., Easterling, M.R., Bucher, J.R., & L.S. Birnbaum. 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment International 83: 107-115. [138] Volkel, W., Colnot, T., Csanady, G.A., Filser, J.G., and W. Dekant. 2002. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chemical Research in Toxicology 15(10): 1281-1287. [139] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138. [140] Serrano, S.E., Braun, J., Trasande, L., Dills, R., and Sathyanarayana, S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health 13: 43. [141] Guart, A., Bono-Blay, F., Borrell, A., and Lacorte, S. 2011. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Additives & Contaminants Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 25(5): 676-685. [142] Bhunia, K., Sablani, S.S., Tang, J., and Rasco, B. 2013. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Comprehensive Reviews in Food Science and Food Safety 12(5): 523-545. [143] Bang, D.Y., Kyung, M., Kim, M.J., Jung, B.Y., Cho, M.C., Choi, S.M., Kim, Y.W., Lim, S.K., Lim, D.S., Won, A.J., Kwack, S.J., Lee, Y., Kim, H.S., and Lee, B.M. 2012. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Comprehensive Reviews in Food Science and Food Safety 11(5): 453-470. [144] Groh, K.J., Geuke, B., and Muncke, J. 2017. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food and Chemical Toxicology 109(1): 1-18. [145] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103. [146] Schecter, A., Lorber, M., Guo, Y., Wu, Q., Yun, S.H., Kannan, K., Hommel, M., Imran, N., Hynan, L.S., Cheng, D., Colacino, J.A., and Birnbaum, L.S. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environmental Health Perspectives 121(4): 473-479. [147] Cariou, R., Larvor, F., Monteau, F., Marchand, P., Bichon, E., Dervilly-Pinel, G., Antignac, J-P., and Le Bizec, B. 2016. Measurement of phthalates diesters in food using gas chromatography-tandem mass spectrometry. Food Chemistry 196: 211-219. [148] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138. [149] Cirillo, T., Latini, G., Castaldi, M.A., Dipaola, L., Fasano, E., Esposito, F., Scognamiglio, G., Di Francesco, F., and Cobellis, L. 2015. Exposure to di-2-ethyhexyl phthalate, di-N-butyl phthalate and bisphenol A through infant formulas. Journal of Agricultural and Food Chemistry 63(12): 3303-3310. [150] Fierens, T., Vanermen, G., Van Holderbeke, M., De Henauw, S., and Sioen, I. 2012. Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology 50(12): 4428-4435. [151] Ionas, A.C., Dirtu, A.C., Anthonissen, T., Neels, H., and Covaci, A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environment International 65: 54-62. [152] Perdue, L. (n.d.-b). Revised Stealth Syndromes Human Study Protocol: APPENDIX 2 – Detailed Parameters of Intervention Diet Selections | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1270 [152] Appendix 3 in the IRB-approved study revision. Microsoft Word – RevisedSSHSprotocol-IRB-CHRApproved-111418-SourceControlDocument.docx (ideaworx.com) [152] Perdue, L. (n.d.-a). Accounting for Unknown Interactions of Co-existing Factors that Confounded Causality Conclusions in Two Studies | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1661 [155] European Food Safety Authority. (2021, December 15). Bisphenol A: EFSA draft opinion proposes lowering the tolerable daily intake. https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake [156] Perdue, L. (n.d.). Basic Scientific Lab Standards — Best Practices for a Replicable Dietary Intervention | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1507 [157] Perdue, L. (n.d.-b). Revised Stealth Syndromes Human Study Protocol: APPENDIX 2 – Detailed Parameters of Intervention Diet Selections | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1270 [158] Perdue, L. (n.d.-b). Black Box Meals: Lessons from Five Dietary Intervention Studies | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1604 [159] Neltner, T.G., Kulkarni, N.R., Alger, H.M., Maffini, M.V., Bongard, E.D., Fortin, N.D., and E.D. Olsen. 2011. Navigating the U.S. Food Additive Regulatory Program. Comprehensive Reviews in Food Science and Food Safety 10(6): 342-368. https://doi.org/10.1111/j.1541-4337.2011.00166.x [160] Barabási, AL., Menichetti, G. & J. Loscalzo. 2020. The unmapped chemical complexity of our diet. Nature Food 1: 33–37. https://doi.org/10.1038/s43016-019-0005-1 [161] Monteiro, C.A., Levy, R.B., Claro, R.M., Ribeiro de Castro, I.R., and G. Cannon. 2010. A new classification of foods based on the extent and purpose of their processing. Cadernos de Saúde Pública 26(11). https://doi.org/10.1590/S0102-311X2010001100005 [162] Monteiro, C., Cannon, G., Moubarac, J., Levy, R., Louzada, M., & Jaime, P. 2018. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutrition, 21(1), 5-17. doi:10.1017/S1368980017000234 [163] Monteiro, C., Cannon, G., Levy, R., Moubarac, J., Louzada, M., Rauber, F., Khandpur, N., Cediel, G., Neri, D., Martinez-Steele, E., Baraldi, L.G., and P.C. Jaime. 2019. Ultra-processed foods: What they are and how to identify them. Public Health Nutrition, 22(5), 936-941. doi:10.1017/S1368980018003762 [164] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138. [165] Serrano, S.E., Braun, J., Trasande, L., Dills, R., and Sathyanarayana, S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health 13: 43. [166] Guart, A., Bono-Blay, F., Borrell, A., and Lacorte, S. 2011. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Additives & Contaminants Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 25(5): 676-685. [167] Bhunia, K., Sablani, S.S., Tang, J., and Rasco, B. 2013. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Comprehensive Reviews in Food Science and Food Safety 12(5): 523-545. [168] Bang, D.Y., Kyung, M., Kim, M.J., Jung, B.Y., Cho, M.C., Choi, S.M., Kim, Y.W., Lim, S.K., Lim, D.S., Won, A.J., Kwack, S.J., Lee, Y., Kim, H.S., and Lee, B.M. 2012. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Comprehensive Reviews in Food Science and Food Safety 11(5): 453-470. [169] Groh, K.J., Geuke, B., and Muncke, J. 2017. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food and Chemical Toxicology 109(1): 1-18. [170] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103. [171] Schecter, A., Lorber, M., Guo, Y., Wu, Q., Yun, S.H., Kannan, K., Hommel, M., Imran, N., Hynan, L.S., Cheng, D., Colacino, J.A., and Birnbaum, L.S. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environmental Health Perspectives 121(4): 473-479. [172] Cariou, R., Larvor, F., Monteau, F., Marchand, P., Bichon, E., Dervilly-Pinel, G., Antignac, J-P., and Le Bizec, B. 2016. Measurement of phthalates diesters in food using gas chromatography-tandem mass spectrometry. Food Chemistry 196: 211-219. [173] Van Holderbeke, M., Geerts, L., Vanermen, G., Servaes, K., Sioen, I., De Henauw, S., and Fierens, T. 2014. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environmental Research 134: 345-352. [174] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138. [175] Cirillo, T., Latini, G., Castaldi, M.A., Dipaola, L., Fasano, E., Esposito, F., Scognamiglio, G., Di Francesco, F., and Cobellis, L. 2015. Exposure to di-2-ethyhexyl phthalate, di-N-butyl phthalate and bisphenol A through infant formulas. Journal of Agricultural and Food Chemistry 63(12): 3303-3310. [176] Fierens, T., Vanermen, G., Van Holderbeke, M., De Henauw, S., and Sioen, I. 2012. Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology 50(12): 4428-4435. [177] Ionas, A.C., Dirtu, A.C., Anthonissen, T., Neels, H., and Covaci, A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environment International 65: 54-62. [178] Perdue, L. (n.d.-d). Revised Stealth Syndromes Human Study Protocol: APPENDIX 2 – Detailed Parameters of Intervention Diet Selections | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1270 [179] Perdue, L., & V. I. Reus (n.d.-d). Revised Protocol: UCSF IRB/CHR Number: 15-17703 Approved by IRB/CHR / http://ideaworx.com/RevisedSSHSprotocol-IRB-CHRApprovead-111418-SourceControlDocument.pdf [180] Perdue, L. (n.d.-b). Accounting for Unknown Interactions of Co-existing Factors that Confounded Causality Conclusions in Two Studies | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=1661 [181] Schwartz, J., Laden, F., & A. Zanobetti. 2002. The concentration-response relation between PM(2.5) and daily deaths. Environmental Health Perspectives 110(10): 1025-1029. [182] Pope III, C.A., Ezzati, M., Cannon, J.B., Allen, R.T., Jerrett, M., & R.T. Burnett. 2017. Mortality risk and PM2.5 air pollution in the USA: an analysis of a national prospective cohort. Air Quality, Atmosphere & Health 11: 245-252. [183] Franklin, M., Zeka, A., & J. Schwartz. 2006. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of Exposure Science & Environmental Epidemiology 17: 279-287. [184] PurpleAir, Inc. (n.d.). PurpleAir | Real-time Air Quality Monitoring. https://www2.purpleair.com/ [185] PurpleAir, Inc. (n.d.-a). Products. https://www2.purpleair.com/products/list [186] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., & B. Lanphear. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384. [187] Dodson, R.E., Nishioka, M., Standley, L.J., Perovich, L.J., Brody, J.G., & R.A. Rudel. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental Health Perspectives 120(7): https://doi.org/10.1289/ehp.1104052 [188] EDC Footprint Calculator (n.d.). https://sites.psu.edu/edccalculator/ [189] Excel EDC Footprint Calculator. (n.d.). https://sites.psu.edu/edccalculator/excel-edc-footprint-calculator/
Additional References
- Hay, M., Thomas, D.W., Craighead, J.L., Economides, C., & J. Rosenthal. 2014. Clinical development success rates for investigational drugs. Nature Biotechnology 32: 40-51.
- BIO-Biomed Tracker study: overall likelihood of approval (LOA) by FDA from Phase I was 9.6%;https://www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf
- Definiens, R. H. M. C. M. O. (2019, October 11). Reconsidering Drug Discovery & Development in the Face of Another Failed Clinical Trial. Drug Discovery and Development. https://www.drugdiscoverytrends.com/reconsidering-drug-discovery-development-in-the-face-of-another-failed-clinical-trial/
- Perdue, L. (n.d.). Mice Are Not People: Why The Most Important Studies Done With Them Ultimately Fail In Humans | Stealth Syndromes Human Study. https://www.stealthsyndromesstudy.com/?p=174
- Allison, D.B., Bassaganya-Riera, J., Burlingame, B., Brown, A.W., le Coutre, J., Dickson, S.L., van Eden, W., Garssen, J., Hontecillas, R., Khoo, C.S.H., Knorr, D., Kussmann, M., Magistretti, P.J., Mehta, T., Meule, A., Rychlik, M., & C. Vogele. 2015. Goals in Nutrition Science 2015-2020. Frontiers in Nutrition 2: DOI 3389/fnut.2015.00026
- Cardona-Morrell, M., Rychetnik, L., Morrell, S.L., Espinel, P.T., & A. Bauman. 2010. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 10 (653): DOI 1186/1471-2458-10-653
- Guash-Ferré, M., Bulló, M., Martínez-González, M.A., Ros, E., Corella, D., Estruch, R., Fitó, M., Arós, F., Wärnberg, J., Fiol, M., Lapetra, J., Vinyoles, E., Lamuela-Raventós, R.M., Serra-Majem, L., Pintó, X., Ruiz-Gutiérrez, V., Basora, J., and J. Salas-Salvadó. 2013. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Medicine 11(164): DOI: 1186/1741-7015-11-164.
- Rohrmann, S, & D. Faeh. 2013. Should we go nuts about nuts? BMC Medicine 11(165): https://doi.org/10.1186/1741-7015-11-165.
- Harnly, J. 2016. Importance of accurate measurements in nutrition research: Dietary flavonoids as a case study. Advances in Nutrition 7(2): 375-382.
- Johnston, B.C., Alonso-Coello, P., Bala, M.M., Zeraatkar, D., Rabassa, M., Valli, C., Marshall, C., El Dib, R., Vernooij, R.W.M., Vandvik, P.O., and G.H. Guyatt. 2018. Methods for trustworthy nutritional recommendations NutriRECS (Nutritional Recommendations and accessible Evidence summaries Composed of Systematic reviews): A protocol. BMC Medical Research Methodoloy 18:162.
- Garza, C., Stover, P.J., Ohlhorst, S.D., Field, M.S., Steinbrook, R., Rowe, S., Woteki, C., and E. Campbell. 2019. Best practices in nutrition science to earn and keep the public’s trust. The American Journal of Clinical Nutrition 109(1): doi: 1093/ajcn/nqy337
- Johnston, B.C., Seivenpiper, J.L., Vernooij, R.W.M., Zeraatkar, D., Bier, D.M., and G.H. Guyatt. 2019. The philosophy of evidence-based principles and practice in nutrition. Mayo Clinic Proceedings: Innovations, Quality, & Outcomes 3(2): P189-199.
- Satija, A., Stampfer, M.J., Rimm, E.B., Willett, W., & F.B. Hu. 2018. Perspective: Are large, simple trials the solution for nutrition research? Advances in Nutrition 9(4): 378-387.
- Ioannidis, J.P.A. 2019. Unreformed nutritional epidemiology: a lamp post in the dark forest. European Journal of Epidemiology 34: 327-331.
- Giovannucci, E. 2019. Nutritional epidemiology: forest, trees and leaves. European Journal of Epidemiology 34: 319-325.
- Lappe, J.M., & R.P. Heaney. 2012. Why randomized controlled trials of calcium and vitamin D sometimes fail. DermatoEndocrinology 4(2): 95-100.
- Ioannidis, J.P.A. 2013. Implausible results in human nutrition research. BMJ 14: doi: 10.1136/bmj.f6698.
- Blumberg, J., Heaney, R.P., Huncharek, M. Scholl, T., Stampfer, M.. Vieth, R., Weaver, C.M. & Zeisel, S.H. 2010. Evidence-based criteria in the nutritional context. Nutrition Reviews 68(8): 478-484.
- Heaney, R.P. 2008. Nutrients, endpoints, and the problem of proof. The Journal of Nutrition 138(9): 1591-1595.
- Heaney, R.P., Weaver, C.M., and J. Blumberg. 2011. EBN (Evidence-Based Nutrition) Ver. 2.0. Nutrition Today 46(1): 22-26.
- Biesalski, H.K., Aggett, P.J., Anton, R., Bernstein, P.S., Blumberg, J., Heaney, R.P., Henry, J., Nolan, J.M., Richardson, D.P., van Ommen, B., Witkamp, R.F., Rijkers, G.T., & I. Zöllner. 26th Hohenheim Consensus Conference, September 11, 2010 Scientific substantiation of health claims: evidence-based nutrition. Nutrition 27(10Suppl): S1-S20.
- De Roos, B., & L. Brennan. 2017. Personalized interventions – A precision approach for the new generation of dietary intervention studies. Nutrients 9(8): 847.
- Halford, J.C.G., Masic, U., Marsaux, C.F.M., Jones, A.J., Lluch, A., Marciani, L., Mars, M., Vinoy, S., Westerterp-Plantenga, M., & D.J. Mela. 2018. Systematic review of the evidence for sustained efficacy of dietary interventions for reducing appetite or energy intake. Obesity Reviews 19(10): 1329-1339.
- Noman, E.A., Al-Gheethi, A.A.S., Talip, B.A., Mohamed, R.M.S.R., Nagao, H., & A.H.M. Kassim. 2018. Xenobiotic organic compounds in greywater and environmental health impacts. Management of Greywater in Developing Countries: 89-108.
- Bornehag, C.G., Lundgren, B., Weschler, C.J., Sigsgaard, T., Hagerhed-Engman, L., & J. Sundell. 2005. Phthalates in indoor dust and their association with building characteristics. Environmental Health Perspectives 113(10): 1399-1404.
- Harmon, J.P. & R. Otter. 2017. Green chemistry and the search for new plasticizers. ACS Sustainable Chemical Engineering 6(2): 2078-2085.
- D. Darbre. 2018. Overview of air pollution and endocrine disruptors. International Journal of General Medicine 11: 191-207.
- Pinkas, A., Goncalves, C.L., & M. Aschner. 2017. Neurotoxicity of fragrance compounds: A review. Environmental Research 158: 342-349.
- Carroll Jr., W.F., Johnson, R.W., Moore, S.S., & Paradis, R.A. 2017. 4-Poly(Vinyl Chloride). Applied Plastics Engineering Handbook: 73-89.
- Kumar, P.S., & E. Gunasundari. 2018. Green chemistry in textiles. Sustainable Innovations in Textile Chemistry and Dyes: 53-73.
- Formaldehyde in your fabrics. (2011, October 26). OEcotextiles. https://oecotextiles.blog/2011/01/04/formaldehyde-in-your-fabrics/
- Senthil Kumar, P., Suganya, S. 2017. Toxic Free Supply Chain for Textiles and Clothing. In: Muthu, S.S. (eds) Detox Fashion. Textile Science and Clothing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-4777-0_1
- Xue, J., Liu, W., & K. Kannan. 2017. Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in textiles and infant clothing. Environmental Science & Technology 51(9): 5279-5286.
- Amutha, K., & K. Saranya. 2017. Sustainability in Fashion and Apparels. WPI Publishing. eBook ISBN 9781351185875
- Koniecki, D., Wang, R., Moody, R.P., & J. Zhu. 2011. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environmental Research 111(3): 329-336.
- Stenmarck, A., Belleza, E.L., Frane, A., & N. Busch. 2017. Hazardous substances in plastics: – ways to increase recycling. Nordic Council of Ministers.
- Harmon, J., & R. Otter. 2018. Green chemistry and the search for new plasticizers. ACS Sustainable Chemical Engineering 6(2): 2078-2085.
- Huang, P.C., Liao, K.W., Chang, J.W., Chan, S.H., & C.C. Lee. 2018. Characterization of phthalates exposure and risk for cosmetics and perfume sales clerks. Environmental Pollution 233: 577-587.
- Kim, J.H., Lee, D., Lim, H., Kim, T., Suk, K., & J. Seo. 2018. Risk assessment to human health: Consumer exposure to ingredients in air fresheners. Regulatory Toxicology and Pharmacology 98: 31-40.
- Noman, E.A., Al-Gheethi, A.A.S., Talip, B.A., Radin Mohamed, R.M.S., Nagao, H., Mohd Kassim, A.H. (2019). Xenobiotic Organic Compounds in Greywater and Environmental Health Impacts. In: Radin Mohamed, R., Al-Gheethi, A., Mohd Kassim, A. (eds) Management of Greywater in Developing Countries. Water Science and Technology Library, vol 87. Springer, Cham. https://doi.org/10.1007/978-3-319-90269-2_5
- D. Darbre. 2018. Overview of air pollution and endocrine disorders. International Journal of General Medicine 11: 191-207.
- Berger, K.P., Kogut, K.R., Bradman, A., She, J., Gavin, Q., Zahedi, R., Parra, K.L., & Harley, K.G. 2018. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. Journal of Exposure Science & Environmental Epidemiology 29: 21-32.
- Bornehag, C.G., Lundgren, B., Weschler, C.J., Sigsgaard, T., Hagerhed-Engman, L, & J. Sundell. 2005. Phthalates in indoor dust and their association with building characteristics. Environmental Health Perspectives 113(10): 1399-1404.
- Patel. 2017. Fragrance compounds: The wolves in sheep’s clothing. Medical Hypotheses 102: 106-111.
- Gluberman, M., Mandia, L. G., Taylor, R. M., & Gall, H. E. 2016. Development of an Endocrine Disrupting Compounds Footprint Calculator. 2016 ASABE Annual International Meeting. https://doi.org/10.13031/aim.20162455889
- Vinas, P., Campillo, N., Pastor-Belda, M., Oller, A., & M. Hernandez-Cordoba. 2015. Determination of phthalate esters in cleaning and personal care products by dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. Journal of Chromatography A 1376: 18-25.